by Piter Kehoma Boll
If you live in tropical and subtropical areas, or even in forested temperate areas, you may have had the “opportunity” to meet a black fly. Those annoying little dipterans sneak toward you and bite you without you even noticing. After they fly away they let a small round red mark on your skin that will itch like hell in the next hours or days. But this is the least comparing to all the damage those annoying flies can cause.
And talking about damage, we will focus on a black fly named Simulium damnosum. It does not have a specific popular name, but I decided to call it the damaging black fly.
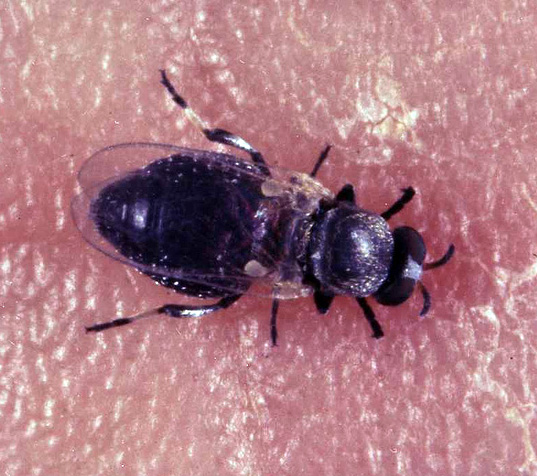
The damaging black fly is very similar to other black flies and it is very hard to differentiate them morphologically. Thus, the name Simulium damnosum is often used in the sense of what is identified as the Simulium damnosum complex, a group of very similar species that can only be differentiated through genetic and cytological aspects. Thus, this article will focus mostly on the complex and not the species Simulium damnosum specifically. They are, afterall, basically identical in all relevant aspects, including their ecology.
Adult damaging black flies measure less than 5 mm in length and have a black chubby body. Females feed on both nectar and mammal blood, while males feed only on nectar. The damaging black fly is found in Subsaharian Africa, especially near rivers. Females lay their eggs in well-oxygenated running water, which larvae need to survive. They are very sensitive to pollution and larvae need immersed substrates, such as rocks or vegetation, to which they anchor themselves using small hooks. Females can lay up to 250 eggs per day and they hatch between 36 and 48 hours after laying.
The life cycle is very fast. The larvae feed on floating organic particles and under favorable conditions turn into pupae after about 8 to 10 days. The pupae remains encased in a coccoon, attached to the substrate and does not move. About three days later the adults emerge from the pupae and move toward the surface, taking flight as soon as they leave the water. The adults mate a few hours after emerging from water, but females continue alive for several days, laying hundred, thousands of eggs, and feeding on mammal blood daily.

As a blood-sucking insect, the damaging black fly is the vector of one of the most serious tropical diseases, onchocerciasis or river blindess. This disease is caused by a nematode that spends part of their life cycle in mammals and part in the flies. Onchocerciasis leads to several debilitating symptoms in humans, especially skin problems and glaucoma, which can cause blindness. It is, in fact, the second most common cause of blindness worldwide, making around 250 thousand people become blind every year.
Since damaing black flies cause such a serious disease to humans and since they live near water courses, such areas are almost uninhabitable in many regions of Subsaharan Africa. However, these areas are the most suitable for agriculture, which causes serious economical problems.
Until now, most attempts to reduce the number of black flies have been unsuccessful. The most effective method until now is the use of inseticides but, as you may guess, this does not only kill the black flies, but many other beneficial insects as well and, as soon as an area is free of black flies, it is colonized again by populations for the surrounding areas. Other alternative to reduce the ability of black flies to reproduce is to build dams that will reduce water flow in rivers, turning the water unsuitable for the larvae, but this, of course, can lead to an even worse ecological disaster.
While the countries affected by the disease lack resources to promote research on the subject, the rich countries couldn’t care less about the human population of such places, as we all know. This is a problem that, in a capitalist world, will hardly find a quick and effective solution.
– – –
– – –
References:
Jacob, B. G., Novak, R. J., Toe, L. D., Sanfo, M., Griffith, D. A., Lakwo, T. L., … & Unnasch, T. R. (2013). Validation of a remote sensing model to identify Simulium damnosum sl breeding sites in sub-Saharan Africa. PLoS Negl Trop Dis, 7(7), e2342.]
Le Berre, R. (1974). Simulium damnosum. In Control of Arthropods of Medical and Veterinary Importance (pp. 55-63). Springer, Boston, MA.
Post, R. J., Onyenwe, E., Somiari, S. A. E., Mafuyai, H. B., Crainey, J. L., & Ubachukwu, P. O. (2011). A guide to the Simulium damnosum complex (Diptera: Simuliidae) in Nigeria, with a cytotaxonomic key for the identification of the sibling species. Annals of Tropical Medicine & Parasitology, 105(4), 277-297.
– – –
* This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
** This work is licensed under a Creative Commons Attribution-Share Alike 2.0 Generic License.
This work is licensed under a Creative Commons Attribution-Share Alike 2.0 Generic License.


















