Hypoxia: Adapt or avoid
All aerobic organisms need oxygen to survive, so they need to be capable of sensing when the amount of oxygen reaching their cells and tissues becomes dangerously low. To cope with hypoxia, organisms activate various protective mechanisms that depend on the duration and/or severity of the oxygen shortage. Understanding how these mechanisms work to detect and combat environments that could deprive an organism of oxygen is a major challenge for researchers.
A large family of enzymes collectively known as protein kinases adds phosphoryl groups to proteins to regulate a wide variety of physiological processes including gene expression, protein activation and protein trafficking. Now, in eLife, Eun Chan Park and Christopher Rongo of Rutgers The State University of New Jersey report that p38 mitogen-activated protein (MAP) kinase is a key component of hypoxic response pathways in neurons of the soil-living nematode, Caenorhabditis elegans (Park and Rongo, 2016).
C. elegans is ideally suited to unraveling the secrets of how neurons protect themselves against hypoxia because its nervous system is rather small, consisting of a total of 302 neurons (White et al., 1986). Additionally, the organization of the nervous system is well-established, as are the molecular pathways that regulate neural circuitry (Macosko et al., 2009). Several toolkits are also available that allow the effects of genetic changes to C. elegans to be easily investigated.
C. elegans responds robustly to changes in oxygen levels (Carrillo and Hallem, 2015). Like in other multi-cellular organisms, the hypoxic response in C. elegans begins with the inhibition of the oxygen sensor EGL-9. This sensor adds hydroxyl groups to proteins that contain the amino acid proline, so when it is inhibited, a transcription factor called HIF-α (hypoxia-inducible factor α) is less likely to be hydroxylated. This, in turn, increases the expression of the genes necessary for adapting to a low-oxygen environment.
Uniquely, hypoxia also induces a behavioral response in C. elegans. In an environment that contains ambient oxygen levels, the nematodes display a random walk pattern of movement, with frequent reversals in direction during long runs of forward motion. However, when they encounter a persistent low-oxygen environment, the frequency with which the nematodes reverse direction is reduced. As a result, the random walk is replaced with a roaming form of motion that allows the nematodes to escape from the low-oxygen environment. This response requires a variant of the EGL-9 oxygen sensor, called EGL-9E, but it does not require HIF-α. The neurons that control locomotion in C. elegans contain a receptor protein called GLR-1 at their synapses. To sustain the random walk behavior, GLR-1 recycling and trafficking to the synaptic region must be maintained.
Studying both normal worms and several “loss-of-function” mutants, Park and Rongo demonstrate that p38 MAP kinase signaling is an integral component of both the transcriptional responses and the behavioral changes that help the nematode to survive hypoxia (Figure 1). Hypoxia, on one hand, inhibits the phosphorylation of EGL-9 by a p38 MAP kinase, thus inhibiting the oxygen sensor and triggering the pathway by which HIF-α increases gene expression. On the other hand, inhibiting p38 MAP kinase initiates roaming-like behavior in the worms because it interferes with the trafficking and recycling of GLR-1. Park and Rongo also show that p38 MAP kinase activity declines with age even when plenty of oxygen is available, which impairs GLR-1 recycling.
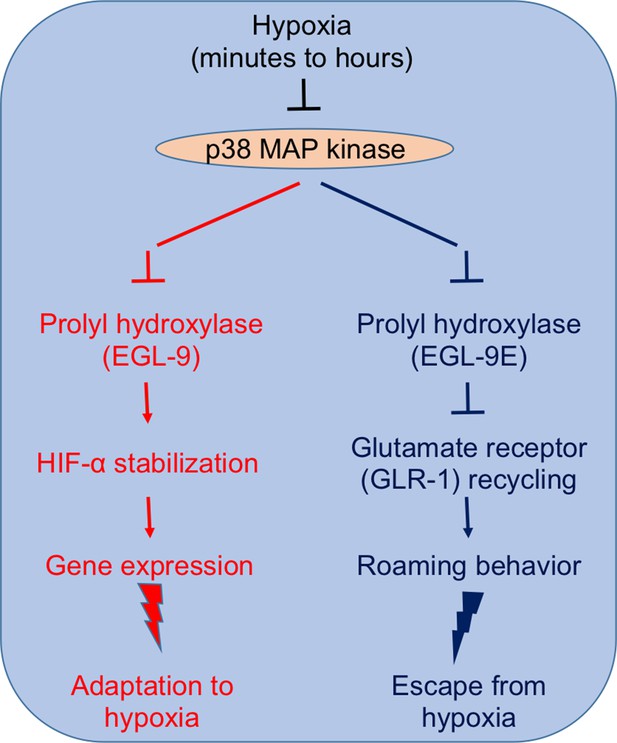
p38 MAP kinase signaling plays a central role in the responses that allow C. elegans to adapt to (left) and avoid (right) prolonged hypoxia (Park and Rongo, 2016).
Left: Inhibiting p38 MAP kinase inhibits the activity of the oxygen sensor EGL-9. This stabilizes the transcription factor HIF-α, which promotes the expression of several genes that help the nematode to adapt to hypoxic conditions. Right: Inhibiting p38 MAP kinase also inhibits EGL-9E (which is an isoform of EGL-9). This causes EGL-9E to dephosphorylate and dissociate from a scaffold protein, which ultimately prevents the recycling of GLR-1 at the synapses of the neurons that control locomotion. The end result is that the worm starts to roam, rather than performing a random walk, which increases its chances of escaping from a hypoxic region.
Previous work investigating acute hypoxia (lasting seconds or minutes) revealed a response that relies on phosphorylation events mediated by two enzymes: soluble guanylate cyclase and protein kinase G (Gray et al., 2004; Yuan et al., 2015). The work of Park and Rongo now suggests that when hypoxia lasts for minutes or hours, varied responses are orchestrated by the EGL-9 oxygen sensor working in concert with different downstream effector molecules. Furthermore, this longer-lasting hypoxia primarily affects the phosphorylation of EGL-9E by p38 MAP kinase. However, the mechanism by which sustained hypoxia affects p38 MAP kinase activity is an open question: does sustained hypoxia directly affect protein thiol groups, which are sensitive to oxidation? Or does it have an indirect effect whereby the generation of reactive oxygen species impacts kinase activity?
References
-
Gas sensing in nematodesMolecular Neurobiology 51:919–931.https://doi.org/10.1007/s12035-014-8748-z
-
The structure of the nervous system of the nematode Caenorhabditis elegansPhilosophical Transactions of the Royal Society B: Biological Sciences 314:1–340.https://doi.org/10.1098/rstb.1986.0056
Article and author information
Author details
Publication history
- Version of Record published: February 23, 2016 (version 1)
Copyright
© 2016, Kumar
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,417
- views
-
- 119
- downloads
-
- 1
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Cell Biology
- Neuroscience
Mutations in Drosophila Swiss cheese (SWS) gene or its vertebrate orthologue neuropathy target esterase (NTE) lead to progressive neuronal degeneration in flies and humans. Despite its enzymatic function as a phospholipase is well established, the molecular mechanism responsible for maintaining nervous system integrity remains unclear. In this study, we found that NTE/SWS is present in surface glia that forms the blood-brain barrier (BBB) and that NTE/SWS is important to maintain its structure and permeability. Importantly, BBB glia-specific expression of Drosophila NTE/SWS or human NTE in the sws mutant background fully rescues surface glial organization and partially restores BBB integrity, suggesting a conserved function of NTE/SWS. Interestingly, sws mutant glia showed abnormal organization of plasma membrane domains and tight junction rafts accompanied by the accumulation of lipid droplets, lysosomes, and multilamellar bodies. Since the observed cellular phenotypes closely resemble the characteristics described in a group of metabolic disorders known as lysosomal storage diseases (LSDs), our data established a novel connection between NTE/SWS and these conditions. We found that mutants with defective BBB exhibit elevated levels of fatty acids, which are precursors of eicosanoids and are involved in the inflammatory response. Also, as a consequence of a permeable BBB, several innate immunity factors are upregulated in an age-dependent manner, while BBB glia-specific expression of NTE/SWS normalizes inflammatory response. Treatment with anti-inflammatory agents prevents the abnormal architecture of the BBB, suggesting that inflammation contributes to the maintenance of a healthy brain barrier. Considering the link between a malfunctioning BBB and various neurodegenerative diseases, gaining a deeper understanding of the molecular mechanisms causing inflammation due to a defective BBB could help to promote the use of anti-inflammatory therapies for age-related neurodegeneration.
-
- Cancer Biology
- Cell Biology
Enhanced protein synthesis is a crucial molecular mechanism that allows cancer cells to survive, proliferate, metastasize, and develop resistance to anti-cancer treatments, and often arises as a consequence of increased signaling flux channeled to mRNA-bearing eukaryotic initiation factor 4F (eIF4F). However, the post-translational regulation of eIF4A1, an ATP-dependent RNA helicase and subunit of the eIF4F complex, is still poorly understood. Here, we demonstrate that IBTK, a substrate-binding adaptor of the Cullin 3-RING ubiquitin ligase (CRL3) complex, interacts with eIF4A1. The non-degradative ubiquitination of eIF4A1 catalyzed by the CRL3IBTK complex promotes cap-dependent translational initiation, nascent protein synthesis, oncogene expression, and cervical tumor cell growth both in vivo and in vitro. Moreover, we show that mTORC1 and S6K1, two key regulators of protein synthesis, directly phosphorylate IBTK to augment eIF4A1 ubiquitination and sustained oncogenic translation. This link between the CRL3IBTK complex and the mTORC1/S6K1 signaling pathway, which is frequently dysregulated in cancer, represents a promising target for anti-cancer therapies.





