Misai Kucing Leaf
Orthosiphon aristatus (Blume) Miq.
Lamiaceae
DEFINITION
Misai kucing leaf consists of dried leaves of O. aristatus (Blume) Miq.
SYNONYM
Orthosiphon stamineus Benth. and Ocimum aristatum Blume [ 1 , 2 ].
VERNACULAR NAMES
Misai kucing (Malay); mao xu cao (Chinese); cat’s whiskers, java tea (English); kumis kucing, remuk jung (Javanese) [ 3 ].
CHARACTER
The dried leaves are green in color with bitter taste and characteristic odour.
IDENTIFICATION
Plant Morphology
It is a herbaceous shrub that can grow up to 1.5 m high. The stem is quadrangular, erect, branching profusely and reddish-coloured. The leaves are arranged in opposite pairs, simple and green; the leaf lamina is oval to lanceolate, glabrous and has serrated margin; the leaf apex is acuminate with acute leaf base; the petioles are short and reddish purple. The inflorescences are campanulate in shape, white or purple in color with long exerted filaments that look like cat’s whiskers [ 3 , 4 ].
Microscopy
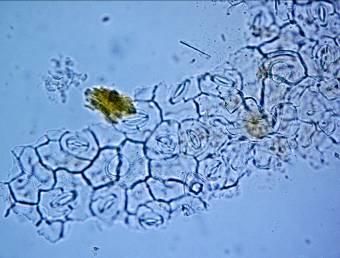
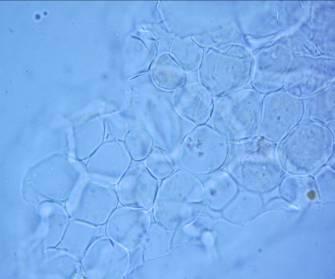
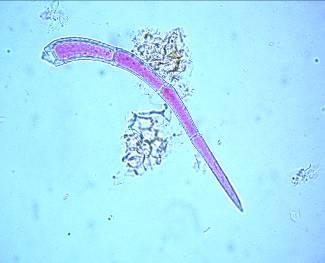
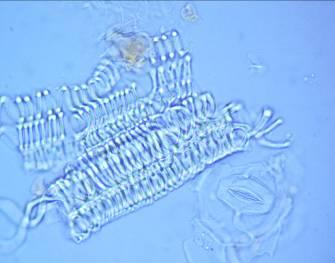
The leaf powder contains fragments of epidermis with sinuous cells, diacytic stomata, parenchymal cells, uniseriate trichomes, fibers, tracheids and fragments of vessels with spiral thickening [ 3 , 4 ].
Colour Tests
Observed colour of solution after treatment with various reagents:
| H2SO4 (conc.) | Brown to dark brown |
| HCI (conc.) | Green to dark green |
Thin Layer Chromatography (TLC)
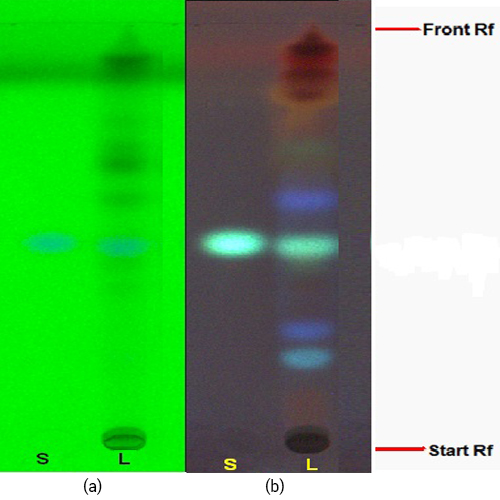
Figure 3 : TLC profiles of sinensetin (S) and ethanol extracts of O. aristatus leaves (L) observed under (a) UV at 254 nm before spraying and (b) UV at 366 nm after spraying with anisaldehyde-sulphuric acid reagent.
| Test Solutions | Weigh about 1.0 g of O. aristatus dried leaves powder in a 50 mL screw-capped conical flask and add 10 mL ethanol into the flask. Sonicate the mixture for 10 min at 60˚C. Cool the mixture and allow the insoluble matter to settle. Filter the mixture and use the filtrate as the test solution. |
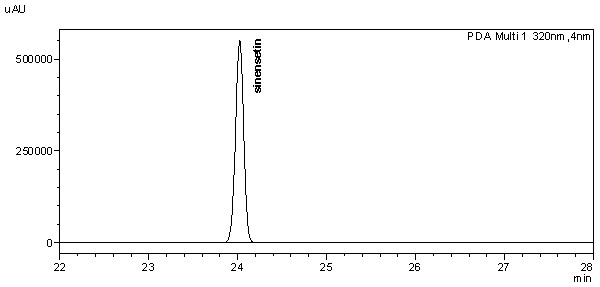
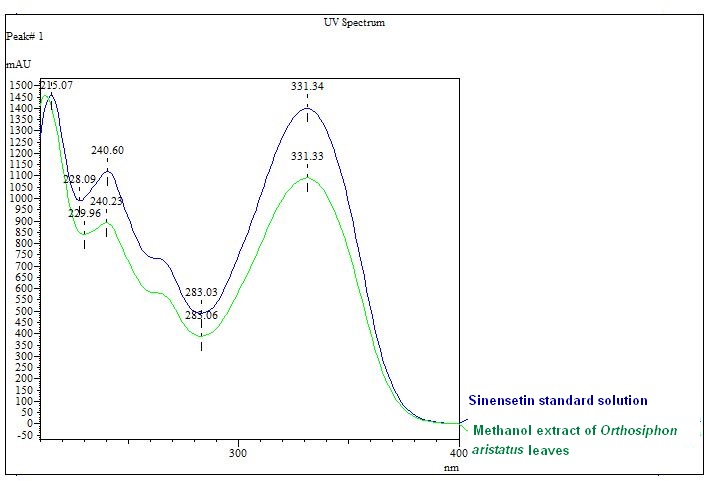
| Standard solution | Dissolve 2.0 mg of sinensetin standard in 20 mL methanol-water (1:1, v/v) to give 100 µg/mL solution. |
| Stationary Phase | HPTLC Silica gel 60 F254, 5 x 10 cm |
| Mobile phase | Hexane: ethyl acetate, 1:4 (v/v) |
| Application |
|
| Development distance | 8 cm |
| Drying | Air drying |
| Detection |
|
High Performance Liquid Chromatography (HPLC)
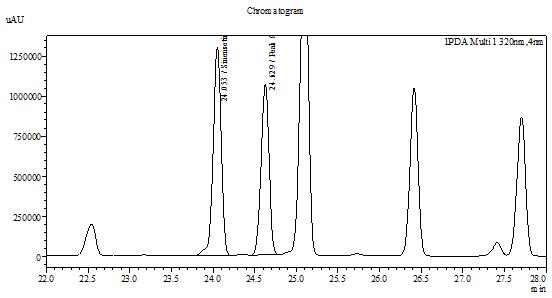
| Test solution | Extract about 1.0 g of O. aristatus dried leaf powder in a 50 mL screw-capped conical flask and add 8 mL of methanol. Sonicate the mixture for 60 mins at 40°C. Cool the mixture and allow the insoluble matter to settle. Filter 3 mL of the upper solution through a 0.45 µm syringe filter and inject the filtrate into the HPLC column. | ||||||||||||||||||
| Standard solution | Dissolve 2.0 mg of sinensetin standard with 20 mL of methanol to produce 100 µg/mL solution. | ||||||||||||||||||
| Chromatographic system |
Detector: UV 320 nm Column: C18 (5 µm, 4.6 mm I.D x 250 mm) Column oven temperature: 30°C Flow rate: 1.5 mL/min Injection volume: 20 µL |
||||||||||||||||||
| Mobile Phase (gradient mode) |
|
||||||||||||||||||
| System suitability requirement |
Perform at least five replicate injections of the sinensetin standard solution (100 µg/mL). The requirements of the system suitability parameters are as follow:
|
||||||||||||||||||
| Acceptance criteria |
|
PURITY TESTS
| Foreign Matter |
| Not more than 2% |
| Ash Contents | |
| Total ash | Not more than 11% |
| Acid-insoluble ash | Not more than 3% |
| Extractive Values | |
| Water-soluble extracts | |
| Hot method | Not less than 27% |
| Cold method | Not less than 18% |
| Ethanol-soluble extracts | |
| Hot method | Not less than 12% |
| Cold method | Not less than 8% |
SAFETY TESTS
| Heavy Metals | |
| Arsenic | Not more than 5.0 mg/kg |
| Mercury | Not more than 0.5 mg/kg |
| Lead | Not more than 10.0 mg/kg |
| Cadmium | Not more than 0.3 mg/kg |
| Microbial Limits | |
| Total bacterial count | Not more than 105 cfu/g |
| Total yeast and mould count | Not more than 104 cfu/g |
| Bile-tolerant gram negative | Not more than 104 cfu/g |
| Specific Pathogens | |
| Salmonella spp. | Absent in 1 g |
| Escherichia coli | Absent in 1 g |
| Staphylococcus aureus | Absent in 1 g |
| Pseudomonas aeruginosa | Absent in 1 g |
CHEMICAL CONSTITUENTS
Aqueous extract of the leaves has been found to contain diterpenes (e.g. neoorthosiphols A and B, orthosiphols A and B, orthosiphonones A and B), flavonoids (e.g. sinensetin, tetramethylscutellarein, 5-hydroxy-6,7,3’,4’-tetramethoxyflavone) and benzochromenes (e.g. methylripariochromene A, acetovanillochromene, orthochromene A) [ 5 , 6 ].
Methanol extract of the leaves has been found to contain flavanoids (e.g. sinensetin, eupatorin, 5-hydroxy-6,7,3’,4’-tetramethoxyflavone, salvigenin, 6-hydroxy-5,7,3’-trimethoxyflavone, 5,6,7,3’-tetramethoxy-4’-hydroxy-8-C-prenylflavone, 3’-hydroxy-5,6,7,4’-tetramethoxyflavone), triterpenes (e.g. ursolic acid, oleanolic acid, betulinic acid, hydroxybetulinic acid, α-amyrin, β-amyrin, maslinic acid, orthosiphonic acid) and caffeic acid derivative (e.g. rosmarinic acid) [ 7, 8 , 9 , 10 ,11 ].
Essential oil of the leaves has been found to contain monoterpenes (e.g. α-pinene, ρ-cymene, 1,8-cineol, limonene, linalool, camphor, δ-terpineol, methylchavicol, borneol, menthone, isomenthone, γ-elemene, α-cubebene, damascenone, α-copaene, eugenol, methyleugenol), sesquiterpenes (e.g. β-carryophyllene, α-humulene, β-elemene, β-bourbonene, cis-caryophyllene, geranylacetone, β-ionone, germacrene D, α-muuiolene, δ-cadinene, germacrene B, dehydroionone, caryophyllene oxide, hexahydrofamesylacetone) and others (hexanal, trans-2-hexanal, cis-3-hexen-1-ol, hexan-1-ol, 4-heptenal, heptenal, benzaldehyde, camphene, 1-octen-3-ol, β-pinene, 3-octanol, 2-pentenylfurane, 2-amylfurane, acetophenone, cis-2-octenal, phenylacetaldehyde, trans,cis-octa-3,5-dien-2-one, cis-linalooloxide, trans,trans-octa-3,5-dien-2-one, trans-linalooloxide, undecan, 2,6,6-trimethyl-2-cyclohexe-1,4-dione, perillen, trans-2-(cis)-6-nonadienale, decanal, naphthalene, dodecane, citronellol, carvone, β-cyclocitral, trans-anethol, isobornylacetate, safranal, 1-methylnaphthalene, bornyl acetate, tridecan, 2-methylnaphthalene, trans,trans-deca-2,4-dienal) [ 12 ].
MEDICINAL USES
Uses described in folk medicine not suported, by experimental or clinical data
This plant is traditionally used to treat tonsillitis, eruptive fever, influenza, menstrual disorders, gonorrhea, syphilis, renal calculus, gallstones, lithiasis, rheumatism, diabetes, hypertension, epilepsy, edema, hepatitis and jaundice [ 13 , 14 ] as well as has diuretic activity [ 15 ]. The tea of O. aristatus leaves is taken as beverage to improve health [ 16 ]. Traditional herbal medicinal product containing O. aristatus leaves has been used as an adjuvant in minor urinary tract complaints [ 17 ].
Biological and pharmacological activities supported by experimental data
Inhibition of calcium oxalate crystal growth
Methanol (50%) extract of O. aristatus leaves (5000 ppm) inhibited the growth of calcium oxalate crystal with an inhibition index of 0.290 after 24 hours of exposure using the modified Scheider’s gel slide method [ 18 ].
Antipyretic activity
Standardized water-methanol (50:50) extract of O. aristatus leaves (up to 1000 mg/kg) given via oral administration for 24 hours to yeast-induced pyrexia Sprague Dawley male and female rats significantly (p<0.05) reduced the body temperature [ 16 ].
Anti-angiogenic activity
Methanol extract of O. aristatus leaves significantly inhibited (p<0.05) the blood vessel growth of rat aortic tissues with an IC50 value of 19.05 µg/mL using rat aorta angiogenesis assay [ 19 ].
Diuretic activity
Methanol extracts of O. aristatus leaves, given to adult male Sprague-Dawley rats (150-250 g) for seven days, were found to increase diuretic effect after 8 hours at a dose of 2 g/kg and urine volume after 2 to 3 days at a dose of 0.5 g/kg [ 20 ].
SAFETY INFORMATION
Preclinical studies (Toxicology studies)
Acute toxicity
Oral single dose acute toxicity study of aqueous mixture of O. aristatus leaf powder on female Sprague Dawley rats (aged between 8 and 12 weeks old) showed no toxic effects on the parameters observed, including behaviours, body weight, food and water intake. All rats were observed for 14 days prior to necropsy. No death was found throughout the study period. Necropsy revealed no significant abnormality. No-observed-adverse-effect level (NOAEL) is more than 2,000 mg/kg body weight [ 21 ].
Standardized ethanolic (50%) extract of O. aristatus leaves (5000 mg/kg) given orally for 14 days to both male and female Sprague-Dawley rats (8 weeks old) did not show any signs of toxicity or death with the LD50 value > 5000 mg/kg [ 22 ].
Sub-acute toxicity
Methanol extract of O. aristatus leaves (up to 5000 mg/kg) given orally to young female Sprague Dawley rats (7±1 weeks old) once daily for 14 days showed significant (p<0.05) decrease in serum AST and ALT levels, and significant (p<0.05) increase in relative liver weight compared to the control with LD50 value > 5000 mg/kg. No signs of toxicity or death were observed in the treated rats [ 23 ].
Sub-chronic toxicity
Standardized ethanolic (50%) extract of O. aristatus leaves (up to 5000 mg/kg) given orally to 8-week old female and male Sprague Dawley rats for 28 days did not showed any mortality or abnormalities in necropsy and histopathogy tests. The haematology and blood chemistry parameters of the treated rats are within the normal range of the normal control groups [ 17 ].
Others (Adverse reaction, contraindication, side effect, warning, precaution)
The use of herbal substance and preparation is contraindicated in people who are hypersensitive to any of the active substances [ 17 ].
The use is not recommended in children and adolescents under 18 years of age due to lack of established data [ 17 ].
During the use of the herbal substance and preparation, appropriate fluid intake is recommended. If complaints of symptoms such as fever, dysuria, spasms or blood in the urine occur, a doctor or a qualified health care professional should be consulted. The use of this product is not recommended in case of oedema due to limited heart and kidney function [ 17 ].
DOSAGE
For adults and elderly [ 17 ]:
1) Dried O. aristatus leaves for tea preparation: 6 to 12 g daily in divided doses.
2) Herbal preparations of O. aristatus leaves:
- Liquid extract: 2 g, 1 to 2 times daily.
- Dry extract (5-7:1): 360 mg, 3 to 4 times daily.
- Dry extract (8-12:1): 200 to 400 mg, 3 times daily.
- Dry extract (7-8:1): 280 mg, 3 times daily.
STORAGE
Store below 30°C. Protect from light and moisture.
REFERENCES
- The plant list. [Internet] Orthosiphon aristatus; 2010 [cited on 30th October 2012]. Available from: http://www.theplantlist.org/tpl/search?q=orthosiphon+aristatus.
- Australian national botanic gardens. [Internet] The Australian plant name index; 2010 [cited on 30th October 2012]. Available from: http://www.anbg.gov.au/cgi-bin/wintab.
- Malaysian herbal monograph. Volume 2. Kuala Lumpur: Forest Research Institute Malaysia. 2009.
- British pharmacopoeia commission. British Pharmacopoeia. London, England: Stationery Office. 2009.
- Ohashi K, Bohgaki T, Matsubara T, Shibuya H. Indonesian medicinal plants. XXIII. Chemical structures of two new migrated pimarane-type diterpenes, neoorthosiphols A and B, and suppressive effects on rat thoracic aorta of chemical constituents isolated from the leaves of Orthosiphon aristatus (Lamiaceae). Chemical and Pharmeceutical Bulletin. (Tokyo). 2000;48(3):433-435.
- Ohashi K, Bohgaki T, Shibuya H. Antihypertensive substance in the leaves of kumis kucing (Orthosiphon aristatus) in Java Island. Yakugaku Zasshi. 2000;120(5):474-482.
- Hossain MA, Mizanur Rahman SM. Isolation and characterisation of flavonoids from the leaves of medicinal plant Orthosiphon stamineus. Arabian Journal of Chemistery. 2011.
- Akowuah GA, Zhari I, Norhayati I, Sadikun A, Khamsah SM. Sinensetin, eupatorin, 3′-hydroxy-5, 6, 7, 4′-tetramethoxyflavone and rosmarinic acid contents and antioxidative effect of Orthosiphon stamineus from Malaysia. Food Chemistry. 2004;87(4):559-566.
- Pietta PG, Mauri PL, Gardana C, Bruno A. High-performance liquid chromatography with diode-array ultraviolet detection of methoxylated flavones in Orthosiphon leaves. Journal of Chromatography A. 1991;547:439-442.
- Hossain MA, Ismail Z. Isolation and characterization of triterpenes from the leaves of Orthosiphon stamineus. Arabian Journal Chemistery. 2010;6(13):295-298.
- Hossain MA, Ismail Z. A new lupene-type triterpene from the leaves of Orthosiphon stamineus. Indian Journal of Chemistery. 2005;44B:436-437.
- Hossain MA, Ismail Z, Rahman A, Kang SC. Chemical composition and anti-properties of the essential oils and crude extracts of Orthosiphon stamineus Benth. Industrial Crops and Products. 2008;27(3):328-334.
- Eisai PT. Indonesia medicinal herb index in Indonesia. Jakarta, Indonesia: Dian Rakyat Publication. 1995;p.263.
- WHO Regional Office for the Western Pacific Manila and Institute of Material Medica Hanoi. Medicinal Plants in Viet Nam; Tran, K., Ed.; Hanoi: Science and Technology Publishing House. 1970.
- Burkill, IH. A dictionary of the economic products of the Malay Peninsula. Malaysia: Ministry of Agriculture. 1966; Vol 2:p. 1592.
- Yam MF, Ang LF, Basir R , Salman IM , Ameer OZ, Asmawi MZ. Evaluation of the anti-pyretic potential of Orthosiphon stamineus Benth. standardized extract. Inflammopharmacology. 2009;17(1):50-54.
- European Medicines Agency. Community herbal monograph on Orthosiphon stamineus Benth., folium; 2010.[cited on 30th October 2012]. Available form: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_Community_herbal_monograph/2011/01/WC500100376.pdf
- Dharmaraj S, Hossain MA, Zhari S, Gam LH, Ismail, Z. The use of principle component analysis and self-organizing map to monitor inhibition of calcium oxalate crystal growth by Orthosiphon stamineus extract. Chemometrics and Intelligent Laboratory. 2006;(81):21-28.
- Sahib HB, Aisha AF, Yam MF, Asmawi MZ, Ismail Z, Salhimi SM, Othman NH, Abdul-Majid AMS. Anti-angiogenic and anti oxidant properties of Orthosiphon stamineus Benth. methanolic leaves extract. International Journal Pharmacology. 2009;5:162-167.
- Arafat OM, Tham SY, Sadikun A, Zhari I, Houghton PJ, Asmawi MZ. Studies on diuretic and hypouricemic effects of Orthosiphon stamineus methanol extracts in rats. Journal of Ethnopharmacology. 2008;118(3):354-360.
- Teh BP, Hamzah NF, Rosli SNS, Yahaya MAF, Zakiah I, Murizal Z. Acute oral toxicity study of selected Malaysian medicinal herbs On Sprague Dawley rats. Institute for Medical Research, Ministry of Health; 2012. Report No.: HMRC 11-045/01/OS/L/P.
- Mohamed EAH, Lim CP, Ebrika OS, Asmawi MZ, Sadikun A, Yam MF. Toxicity evaluation of a standardised 50% ethanol extract of Orthosiphon stamineus. Journal of Ethnopharmacology. 2011;133(2):358-363.
- Chin JH AH, Sabariah I. Toxicity study of Orthosiphon stamineus Benth (misai kucing) on Sprague Dawley rats. Tropical Biomedicine. 2008;25(1):9-16.