Abstract
A fossil leaf compression from the Late Oligocene (28–27 Ma) of northwestern Ethiopia is the earliest record of the African endemic moist tropical forest genus Cola (Malvaceae sensu lato: Sterculioideae). Based on leaf and epidermal morphology, the fossil is considered to be very similar to two extant Guineo-Congolian species but differences warrant designation of a new species. This study also includes a review of the fossil record of Cola, a comprehensive summary of leaf characteristics within several extant species of Cola, Octolobus, and Pterygota, and a brief discussion of the paleogeographic implications of the fossil species affinity and occurrence in Ethiopia.
Similar content being viewed by others
Introduction
The Malvaceae sensu lato (which includes Bombacaceae, Malvaceae sensu stricto, Sterculiaceae, and Tiliaceae) are major woody components of present-day African tropical moist forests (Judd and Manchester 1997; Alverson et al. 1999). For example, in most 0.1 ha African forest plots examined by Gentry (1988), the Sterculiaceae or Tiliaceae were almost always among the top ten most species-rich angiosperm families (Phillips and Miller 2002). In these plots, the most speciose group within Malvaceae s.l. is a subclade of the Cola clade sensu Wilkie et al. (2006) consisting of Cola Schott & Endl., Octolobus Welw., and Pterygota Schott & Endl.
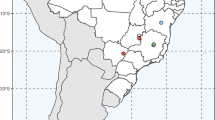
The African endemic Cola, the second largest genus (approximately 100–125 species) in the subfamily Sterculioideae, is almost entirely restricted to moist evergreen and semideciduous forest formations (Cheek 2002; Bayer and Kubitzki 2003; Judd et al. 2008). The genus, which typically is a common component of the forest understorey, includes shrubs, treelets, and trees that rarely reach more than 30 m in height. While the majority of Cola species are found in West and Central Africa, about 20 species occur in eastern (Uganda, Tanzania, Kenya, and southern Somalia), northern (Mali, Niger, and southern Sudan), and southern Africa (Malawi, Mozambique, South Africa, and Zambia) (Fig. 1; Keay and Brenan 1973; Verdoorn 1981; White et al. 2001; Cheek 2002; Lebrun and Stork 2003; Cheek personal communication).
Distribution map of the genus Cola, modified from Lebrun and Stork (2003) and Coates Palgrave (1977). Geometric shapes correspond to the number of species that occur (or co-occur) within a particular area: circles 1 or 2 species, triangles 3–5 species, squares 6–9 species; pentagons, 10 or more species. Daggers denote confirmed and purported fossil records of Cola
Octolobus is closely related, and possibly sister to Cola (Cheek and Frimodt-Møller 1998; Wilkie et al. 2006). This African endemic tri- or tetratypic genus consists of small forest trees found mainly in West and Central Africa, although one species reaches Tanzania (Cheek and Frimodt-Møller 1998; Bayer and Kubitzki 2003). Both genera are similar in possessing indehiscent follicles and exalbuminous seeds (Bayer and Kubitzki 2003; Wilkie et al. 2006). Pterygota, a pantropical genus of about 15 species of tall forest trees, differs from both Cola and Octolobus in possessing dehiscent follicle fruits with winged seeds (Mabberley 1997; Bayer and Kubitzki 2003; Lebrun and Stork 2003).
A recently discovered fossil leaf compression from the Late Oligocene of Ethiopia (28–27 Ma) Guang River flora can be identified confidently as Cola and is described below. This fossil represents the oldest record of the genus. The presence of a fossil Cola from Ethiopia is interesting biogeographically because the genus is not present there today (Friis 1992; Vollesen 1995) and indicates a different distribution on the Afro-Arabian continent during the Paleogene (Fig. 1). In addition, the genus is an indicator of moist forest communities and thus provides insight into the Late Oligocene vegetation on the northwestern Ethiopian plateau.
Very little information on the epidermal anatomy of living Cola has been published and no data are available regarding the leaf epidermal anatomy of Octolobus or Pterygota. The only sources of data about epidermal micromorphology within Cola are Gehrig (1938), Metcalfe and Chalk (1950), and Inamdar et al. (1983), none of which are comprehensive. Consequently, leaf cuticle samples from several extant species of Cola and some species of Octolobus and Pterygota were prepared, examined, and described to provide comparative epidermal micromorphological characters. These data have proven useful in identification of the fossil and are provided here for future systematic and phylogenetic investigations of the genus and the Sterculioideae (Table 1 and Appendix).
Materials and methods
Cuticle was prepared from fossil leaf fragments (0.5–1 cm2) by first rinsing the material in 10% hydrochloric acid (HCl) for 10–30 min to remove carbonates. The leaf material was then rinsed with distilled water and placed in a solution of 48% hydrofluoric acid (HF) for 24–48 h to dissolve the silicate matrix. Following this step, the cuticle was placed in a 20% bleach (NaClO)–water solution for a very brief amount of time (on the order of 20 s to 1 min) to dissolve away excess organics. The cuticle was thoroughly rinsed with distilled water and mounted on microscope slides in glycerine.
Leaf cuticle samples from 38 extant species of Cola, 2 species of Octolobus, and 2 species of Pterygota were sampled for comparative purposes from herbaria at the Missouri Botanical Garden (MO) and the Royal Botanic Gardens at Kew (K). Samples (0.5–1 cm2) were soaked in distilled water for 24 h to resaturate. Subsequently, the specimens were treated with a solution of 10% potassium hydroxide (KOH) until they darkened fully, washed with distilled water, and placed in baths of 30–60% bleach to remove mesophyll. The cleared specimens were then stained with a Safranine O solution, rinsed with 90% ethanol (C2H5OH), and mounted on microscope slides in glycerine jelly. Light microscope slides of both the fossil and herbarium samples are housed in the Roy M. Huffington Department of Earth Sciences at Southern Methodist University in Dallas, TX.
Most of the terminology and definitions used in the text and Appendix are taken from the Manual of Leaf Architecture (Ellis et al. 2009) and Dilcher (1974). Where terms differ, they are defined in the text. Dominant abaxial and adaxial trichome characters (characters 26 and 41 in Table 1 and Appendix) were determined by randomly observing 100 hairs among the prepared cuticle microscope slides of each species. In cases where fewer than 100 hairs were present on a slide, trichome dominance was based on a percentage of fewer than 100 randomly observed hairs. If the number of hairs observed was fewer than 20, and there were more than two hair types present the character state was scored as “no trichomes predominate.” Epidermal cell sizes are recorded in measured micrometer ranges (µm; characters 14 and 29 in Table 1, Appendix). For these measurements only nonsubsidiary epidermal cells that were not adjacent to veins or hairs were included.
The Guang River flora
The newly discovered Ethiopian Late Oligocene (28–27 Ma) fossil is from a moderately diverse assemblage (approximately 40 morphotypes, assumed to be species) of well-preserved leaf, floral, and wood (logs and stumps) compressions, known as the Guang River flora (Pan et al. 2006; Pan 2007). The flora is located in the northwestern plateau region of Ethiopia, about 60 km west of Gondar (in the Chilga Woreda, Amhara region; Fig. 1). Rocks of this region consist of massive Oligocene trap basalts interspersed with tuffs, lignites, and fluvial volcaniclastic and clastic sediments exposed along streams and gullies (Mohr 1971; Yemane et al. 1987; Kappelman et al. 2003; Jacobs et al. 2005). Along a portion of the Guang River, a 100 m sedimentary section has been dated to between 28 and 27 Ma (Chron C9n) based on K–Ar and 40Ar/39Ar radiometric dates and paleomagnetic reversal stratigraphy (Kappelman et al. 2003; Cande and Kent 1995). Fossils are prevalent in these sediments and include plants, mammals, and invertebrates (Kappelman et al. 2003; Sanders et al. 2004; Pan et al. 2006; García Massini et al. 2006).
The autochthonous or parautochthonous Guang River flora is preserved in an approximately 22-cm-thick massive greenish-gray mudstone layer derived from a weathered overbank (or pond) ash deposit, which provided the parent material for an overlying Gleysol (Jacobs et al. 2005; Pan et al. 2006; Pan 2007). The fossiliferous sediment is underlain by a hard 1-cm-thick lignitic layer and overlain by a 70-cm-thick yellowish-green silty claystone. Gleysols are commonly formed in areas with high water tables that are waterlogged for most of the year; lignites are derived from organically rich, waterlogged soils characterized by chemically reducing conditions (Mack et al. 1993). Based on the sediments in which the fossils are preserved, the large number of entire-margined notophyllous to mesophyllous leaf taxa present, species composition, and the high species heterogeneity over short distances (based on lateral sampling), the flora likely represents a moist tropical forest community growing in swampy or water-saturated (at least for a large portion of the year) conditions.
Results
Systematic Paleobotany
Malvaceae Juss. 1789
Sterculioideae Burnett 1835
Cola Schott & Endl. 1832
Cola amharaensis sp. nov. A. D. Pan and B. F. Jacobs
Diagnosis
Entire-margined, mesophyllous leaf with cuneate base, apical pulvinus, and triplinerved basal venation (Fig. 2). Primary venation is pinnate, secondary venation is brochidodromous, and tertiary venation is alternate percurrent with admedially ramified veins (Fig. 2). Abaxial leaf epidermal cells are rectangular and isodiametric (Figs. 3, 4). Stomatal complexes are brachyparacytic and restricted to the abaxial side of the leaf (Fig. 3). Anticlinal cell walls are rounded to undulate and have bead-like thickenings. Periclinal cell walls have a stippled texture (Figs. 3, 4). Abaxial glandular hairs possess globose or capitate heads and are uniseriate or biseriate and multicellular (Fig. 4). Buttressed hair bases are present on the adaxial surface.
Cola amharaensis Pan and Jacobs sp. nov. and Cola flavo-velutina. Fig. 2. Cola amharaensis Holotype CH41-P36/CH41-P41; scale bar 15 mm. Fig. 3. Brachyparacytic stomata of C. amharaensis; scale bar 30 µm. Fig. 4. Multicellular glandular hairs of C. amharaensis; scale bar 30 µm. Fig. 5. Leaf of Cola flavo-velutina; scale bar 15 mm. Fig. 6. Brachyparacytic stomata of C. flavo-velutina; scale bar 30 µm. Fig. 7. Multicellular glandular hairs of C. flavo-velutina; scale bar 30 µm
Description
Mesophyllous leaf compression fragment (and counterpart) with length of 78 mm and maximum width of 58.5 mm. The entire-margined lamina is either elliptic or obovate and symmetrical. The base is cuneate and has an acute angle. The petiole has an apical pulvinus (4 mm long, 2.5 mm wide) which is attached at the top of a 13-mm-long petiole fragment (Fig. 2). The primary venation is pinnate, although the leaf is triplinerved at the base with secondary veins (oriented at more acute angles than subsequent secondaries) following parallel to the margin, thinning toward a connection to the next pair of secondaries (Fig. 2). Secondary venation is brochidodromous and the spacing between pairs of secondary veins decreases somewhat towards the base. Weak intersecondaries are present and merge with the tertiary veins. Tertiary venation is alternate percurrent with admedially ramified vein course. Quaternary veins form a regular polygonal reticulate pattern. Cuticle: Abaxial cells are isodiametric to rectangular in shape and are 16–53 µm long and 10–36 µm wide (Fig. 3). Abaxial anticlinal cell walls are generally straight to rounded and possess bead-like thickenings. Abaxial epidermal periclinal cell surfaces are stippled. The stomatal complex is brachyparacytic and stomata are restricted to the abaxial leaf surface (Fig. 3). Glandular trichomes are present and consist of a single foot cell and uniseriate or biseriate multicellular, generally 3–4 layered, globose or capitate head (Fig. 4). The glandular trichomes range in size from 29 to 35.5 µm long and from 32 to 42 µm wide (Fig. 4), typically wider than long. Adaxial cells are isodiametric, 24–42 µm long and 15–31 µm wide. The adaxial epidermal cell surface ornamentation is similar to that of the abaxial side. Anticlinal cell walls are rounded to undulate. Trichome bases, which may have buttresses, have been observed on the adaxial surface and, based on morphology, likely represent glandular hairs (Fig. 17).
Holotype
CH41-P36/CH41-P41 (Fig. 2); found at sublocality CH41 in the Guang River flora. This specimen is housed at the National Museum of Ethiopia in Addis Ababa.
Etymology
The species is named for the Amhara Province of Ethiopia where the fossil and the Guang River flora were found.
Remarks
The fossil can be placed in the genus Cola based on the following characteristics: simple leaves with entire margins, cuneate base with an acute angle and triplinerved veins, the presence of a pulvinus at the apex of the petiole (Figs. 2, 5), weak brochidodromous secondary venation, and the presence of multicellular glandular hairs on the epidermal surfaces of the leaf (Figs. 4, 7). The fossil differs from Octolobus in possessing a cuneate leaf base and trichomes that are solely glandular. Also, Octolobus spectabilis has stomatal complex chains and clusters (Fig. 23), defined here as four or more adjacent stomatal complexes lacking separating epidermal (nonsubsidiary) cells, a characteristic that is absent in the fossil and almost all extant species of Cola observed, excluding C. letouzeyana. The fossil differs from Pterygota, which have subcordate/cordate, rounded, or almost truncate bases.
Based on leaf and epidermal characters of the species, the fossil most closely resembles Cola attiensis and C. flavo-velutina (Fig. 5), two extant West and Central African species. The fossil is similar to both in (1) possessing a simple-entire margined leaf with a cuneate base, (2) triplinerved basal veins which fade towards the margin, (3) straight to rounded abaxial anticlinal cell walls (Fig. 2), (4) brachyparacytic stomata (or rarely other paracytic stomatal complex types) restricted to the abaxial side (Fig. 3), (5) multicellular glandular hairs present and dominant (≥70% of hairs encountered are glandular) on the abaxial surface (Fig. 4), and (6) adaxial anticlinal cell walls that are rounded to undulate. In addition, hair bases similar to those of the multicellular glandular hairs of the abaxial side are present on the adaxial surface. These bases may indicate that glandular hairs are present and possibly predominant on the adaxial cell surface of the fossil, a characteristic that also occurs within Cola attiensis and C. flavo-velutina. Furthermore, the length and width ranges of abaxial and adaxial epidermal cells in the Ethiopian Cola are quite similar those in C. attiensis and C. flavo-velutina.
Cola attiensis is a small tree or shrub found along river banks or moist ground in evergreen forest growing on sandy clays in Côte d’Ivoire and has been reported from Cameroon and Gabon (Hallé 1961; Lebrun and Stork 2003; Holmgren et al. 2004). Cola flavo-velutina is a shrub or tree growing in the understorey of very moist rainforest in Ghana, Nigeria, Cameroon, and Gabon (Hallé 1961; Lebrun and Stork 2003).
While the fossil differs little from Cola attiensis and C. flavo-velutina based on the characters mentioned above, the fossil is placed in a new species Cola amharaensis because it differs by having buttresses on some adaxial hair bases (Fig. 17; buttresses were absent from all hair bases observed in C. attiensis and C. flavo-velutina), an absence of stellate/peltate hairs or hair bases on the abaxial surface (stellate hairs are present in Cola flavo-velutina), and a lack of straight anticlinal cell walls on the adaxial surface (which can occur within C. attiensis). The closest resemblance to Cola amharaensis sp. nov. among the eastern African species observed in this study is C. usambarensis, which differs from the fossil in having a convex leaf base and possessing only stellate/peltate hairs on its leaf surfaces.
Other eastern and southern Cola species not included in this study are not similar to the fossil (Keay and Brenan 1973; Brenan 1978; Verdoorn 1981; Coates Palgrave 1977; White et al. 2001; Cheek 2002). Primary differences include leaf base morphology (Cola chlorantha, C. congolana, C. gigantea, C. lukei, and C. octoloboides), and the absence of triplinerved or trinerved venation at the base of the leaf (C. congolana).
Comparative leaf morphology in extant Cola, Octolobus, and Pterygota
A variety of leaf types are present within the Cola + Octolobus + Pterygota clade sensu Wilkie et al. (2006). These include both simple and palmately compound leaved species, which are entire-margined, and may be unlobed or lobed. Simple, unlobed, entire-margined leaves are often trinerved (or triplinerved). Cola, a relatively large genus, has a diverse array of leaf morphologies, while Octolobus consists solely of entire-margined, unlobed, simple-leaved species. Octolobus spectabilis is usually not trinerved at the base, but trinerved basal veins do occur consistently within O. heteromerus and O. zenkeri. Pterygota species can have either lobed or unlobed leaves, are usually palmately veined, and often have a cordate or subcordate base, although nearly truncate bases may also occur.
Micromorphology
Epidermal cells
Most epidermal cells within all three genera are isodiametric or rectangular in shape and 3–8 sided (Fig. 8). Abaxial epidermal cells range in size from 8 to >100 µm long and 6 to 48.5 µm wide. Adaxial cells range from 65 to 85 µm in length and 3 to 51.5 µm in width. Species typically have anticlinal cell walls that are either straight to rounded, or rounded to undulate in shape. If undulate, the sinuous walls are widely to narrowly U-shaped (Fig. 8). Fine to prominent stippled ornamentation is present on the abaxial and adaxial periclinal cell walls of almost all species of Cola and Octolobus that we observed (Fig. 8). Stippled ornamentation also occurs in Pterygota macrocarpa. Striations are present on the abaxial and adaxial (though slightly less defined) periclinal cell walls of Pterygota bequaertii (Fig. 9) and abaxially in Cola lepidota. Anticlinal cell wall thickenings are present on all species observed, the most common types being “knob-like” (Fig. 2) and “T-shaped,” which are defined and illustrated in Dilcher (1974).
Leaf epidermal micromorphology; scale bars 30 µm. Fig. 8. Stippled ornamentation on the adaxial epidermal cells of Cola flavo-velutina. Fig. 9. Striated epidermal abaxial surface and silica bodies of Pterygota bequaertii. Fig. 10. Stellate hair on the abaxial surface of Cola mahoundensis. Fig. 11. Stellate or peltate remnant hair base on the abaxial of C. minor. Fig. 12. Multicellular glandular hair on the abaxial surface of C. mahoundensis. Fig. 13. Twinned or coupled glandular hairs on the abaxial surface of C. acuminata. Fig. 14. Abaxial unicellular simple trichome of C. mahoundensis. Fig. 15. Adaxial stone cells of C. buntingii
Trichomes
A variety of different trichome (hair) types occur within the Malvaceae s.l. Although the family is often known for stellate, peltate, and tufted trichomes (Figs. 10, 11; Metcalfe and Chalk 1950; Bayer and Kubitzki 2003; Judd et al. 2008), other types are also common. These include clavate or capitate (globose) multicellular glandular (Figs. 12, 13), unicellular glandular, and simple hairs (Fig. 14). Trichome types found within Cola include glandular, simple unicellular, and stellate/peltate hairs (Figs. 10, 12, 13, 14; in most cases a polygonal base is usually the only indication of the presence of stellate/peltate hairs; Fig. 11). Glandular hairs and stellate/peltate hair bases were observed in all Octolobus and Pterygota species examined; however, simple trichomes were absent among observed species within these two genera.
Stellate/peltate hairs
The majority of species have deciduous stellate/peltate hairs with only polygonal (usually pentagonal or hexagonal)-shaped hair bases with thickened margins and acute points present (Fig. 11). However, whole stellate/peltate trichomes are abundant on the abaxial surfaces of Cola cordifolia and C. lepidota. Intact hairs also occur more sporadically in Cola heterophylla (abaxial), C. lateritia (adaxial), C. mahoudensis (abaxial; Fig. 10), C. millenii (abaxial), and Pterygota bequaertii (adaxial).
Glandular hairs
Glandular hairs were observed in almost every species of Cola, Octolobus, and Pterygota examined with the exception of Cola minor, C. natalensis, and C. usambarensis (Table 1; Appendix). Hair bases are often thickened and can exhibit buttresses, as in Cola ballayi, C. discoglypremnophylla (Fig. 16), and C. simiarum. Glandular hairs emerge from a unicellular rectangular base cell (Fig. 12). Most glandular hairs occur singly, but twinned glandular hairs can be found in Cola acuminate (Fig. 13), C. discoglypremnophylla, C. letouzeyana, and Octolobus zenkeri.
Leaf epidermal micromorphology cont. Abaxial surfaces; scale bars 30 µm. Fig. 16. Buttressed hair base on the abaxial surface of Cola discoglypremnophylla. Fig. 17. Buttressed hair base on the adaxial surface of Cola amharaensis Pan sp. nov. Holotype CH41-P36/CH41-P41. Fig. 18. Brachyparacytic stomata (a paracytic stomatal complex type) of C. uloloma. Fig. 19. Anomocytic stoma (a polycytic stomatal complex type) of C. ballayi. Fig. 20. Tetracytic stomata of C. ballayi. Fig. 21. Hexacytic stoma of C. nitida. Fig. 22. Thickened periclinal wall surrounding the stomata of C. lissachensis. Fig. 23. Stomatal complex cluster of Octolobus spectabilis
Simple hairs
Simple hairs are quite rare within Cola and were only observed in C. mahoundensis (Fig. 14), C. millenii, and C. scheffleri. While it should be noted that Inamdar et al. (1983) reported occasional simple hairs in Cola acuminata, none were observed in this study.
Silica bodies
Silica bodies were observed in several species of Cola, particularly around the veins (Fig. 15). When silica bodies were observed, the majority were found on the abaxial surface of the leaf. Cola acuminata is unusual in having silica bodies both abaxially and adaxially, but they are noticeably more numerous on the abaxial surface.
Silica bodies resemble jagged spheres and are usually on the order of 3–4 µm in diameter (Figs. 9, 15).
Stomata
Nearly all stomata found within Cola, Octolobus, and Pterygota are restricted to the abaxial leaf surface, however a few isolated stomata occur on the adaxial surface in Cola chlamydantha, C. discoglypremnophylla, C. heterophylla, C. hispida, and C. porphyrantha. The majority of species have brachyparacytic and paracytic stomatal complexes (Figs. 3, 16, 18; however, tetracytic (Fig. 20) and anisocytic types are not uncommon and can be prevalent in some species (Fig. 21; Table 1). Rarer stomatal complex types including hemiparacytic, cyclocytic, hexacytic, and anomocytic types can be found among a few species (Figs. 19, 21; Table 1).
Discussion
Fossil record
The fossil record, besides the Guang River flora Cola sp. leaf compression, includes a flower and possibly a leaf cast from the Early Miocene of Uganda, fossil wood of the form genus Colaxylon from the Late Neogene of Ethiopia and Chad, a fossil denoted as cf. Cola from the Early Miocene of Kenya, and several species of Cola and Octolobus reported from the Late Neogene of Cameroon (Fig. 1, Menzel 1920; Hamilton 1968; Koeniguer 1973; Lemoigne 1978; Jacobs and Winkler 1992). A fossil seed attributed to cf. Cola from the Cretaceous of Senegal (Monteillet and Lappartient 1981) does not appear to be similar to the extant genus.
Fossil wood of the form genus Colaxylon is reported by Koeniguer (1973) and Lemoigne (1978) from the Late Neogene of Chad and Ethiopia, respectively. While it is possible that these fossil woods may have affinity to Cola, many genera within the Sterculioideae have similar wood anatomical structures (Metcalfe and Chalk 1950).
The fossil flower from the Bukwa locality in western Uganda is Early Miocene (19.5–19.1 Ma based on 40Ar/39Ar radiometric dating of lavas; Maclatchy et al. 2006). Hamilton (1968) considered the floral cast to have affinities with either Cola or Pterygota based on the near-sessile attachment of two whorls of bilobed anthers to the staminal tube (androgynophore), and the presence of five partially fused sepals. In the illustration provided by Hamilton (1968: Fig. 1a), a structure at the apex of the androgynophore appears to be 4–5 vestigial fused carpels as occurs on male flowers in species of Cola and Pterygota. Hamilton (1968), who compared the fossil to living Ugandan Cola and Pterygota species, considered it to be most similar to Cola gigantea A. Chev., which is found only in Uganda and is often confused with Cola cordifolia, a species restricted to western Africa (Lebrun and Stork 2003). However, in our opinion the fossil’s loose interdigitating whorls of stamens compares more closely to the genus Pterygota, rather than Cola (Hallé 1961 Bodard 1962; Germain 1963).
A lobed fossil leaf from the same bed as the flower was noted by Hamilton (1968) as similar to either Euphorbiaceae or Sterculiaceae (e.g., Cola). Hamilton (1968) notes in his description that each of the numerous main veins (6–10?) leads to a leaf-lobe. However, Cola and Pterygota species (that possess lobed, palmately veined, simple leaves) generally have 3–5 lobes, and Cola gigantea leaves are either unlobed or consistently possess three-lobes. Thus, the affinity of this fossil leaf remains uncertain.
Cf. Cola fossil leaf impressions from the Early Miocene Ngorora Formation (Fig. 24) have weak brochidodromous secondary venation, alternate percurrent tertiary venation, and possibly swollen pulvini, all features common to extant Cola species. Moreover, the shallowly subcordate and slightly asymmetrical base in association with both pinnate basal secondary venation and obovate leaf shape is not common within Cola. Only one species, Cola letouzeyana from Cameroon, is similar in all these respects to the fossil (Nkongmeneck 1985) and also compares favorably in having a decrease in secondary vein angle and spacing towards the leaf base. The fossil also looks superficially similar to some species of Scaphopetalum Mast. (Malvaceae sensu lato: Byttnerioideae), for example S. thonneri De Wild. & Th. Durand; however, it lacks the distinctive trinerved venation arising from the base.
More than 700 specimens of leaf impressions found in tuffs near Mount Cameroon were reported by Menzel (1920), and among these he reports close affinities with 15 extant Cola and two Octolobus species. Menzel (1920) considered all of these impressions to represent taxa that still exist in the area today. Many of these fossils have venation similar to that of Cola and Octolobus; however, definitive identification cannot be established at this time. While the age of these fossils is not known (considered Late Neogene in age according to Menzel 1920), these fossils are preserved in tuff sediments so radiometric dating might be possible. It is interesting to note that today this area of West Africa (the Lower Guinea center) has the highest diversity and species richness of Cola in Africa (Cheek et al. 1996; Cheek 2002) and is considered by Hamilton (1976) to have been a major rainforest refugium during the Pleistocene (Cheek et al. 1996; Linder 2001).
Leaf characters and relationships within Cola
Cola has often been split into a number of subgroups based on leaf and reproductive characteristics by several authors, most of which differ (at least somewhat) from one another (see Bodard 1962 for a summary of taxonomic modifications within the genus). In this paper we have provided leaf and epidermal characters for several species of Cola, Octolobus, and Pterygota (summarized in Table 1 and Appendix).
A number of purported subgenera were erected by Hallé (1961) to categorize Gabon Cola species. One of these, Cola subgenus Neocourtenia (which includes C. gabonensis, C. heterophylla, C. hispida, C. mahoundensis, C. marsupium, and C. urceolata), is characterized by palmately veined, simple-leaved (often lobed) species with inconspicuous tertiary and higher-order veins (only visible in dried leaves), caducous stipules, flowers that are generally less than 2 cm in diameter possessing a uniseriate whorl of stamens on the androecium, stamens numbering one or two per carpel, panicle-like cyme inflorescences, and seeds with a thick, very fibrous integument (Hallé 1961). Within our study, we observed that charactaceous leaf texture also supports close relationship amongst these taxa (of the species listed above, C. urceolata was not examined). Other species (Cola carcifolia, C. millenii, and C. scheffleri) which share some morphological characteristics, including palmately veined simple leaves with lobes and chartaceous texture, with Hallé’s (1961) subgenus Neocourtenia may belong to (or closely related to) the subgenus (Keay and Brenan 1973). Bodard (1962) considered Cola carcifolia and C. millenii to be closely related and placed them in subgenus Haplocola along with C. triloba (R. Br.) K. Schum., C. laurifolia, C. humilis (which is considered a synonym of C. heterophylla; Lebrun and Stork 2003), and C. reticulata (A. Chev.).
Cola chlamydantha, C. lepidota, C. lissachensis, and C. pachycarpa appear to be closely related based on the palmately compound leaves, the possession of anisocytic stomatal complex types, thickened rims (?papillae) on the periclinal wall of the subsidiary cells adjacent to the guard cells (often encircling the stoma), and subsidiary cells with striate ornamentation (Fig. 22). However, Cola chlamydantha and C. lastoursvillensis (M. Bodard & Pellegr.) N. Hallé (not sampled) have often been placed in a separate genus Chlamydocola K. Schum. based on a suite of characters which is unique within the genus Cola including androphore with anthers arranged in a subsinuous or digitate wreath formation, bisexual flowers with 9–12 carpels having numerous (22–26) ovules, albuminous seeds, and large orbicular bracteoles surrounding the flower bud (Hallé 1961; Bodard 1962; Germain 1963; Keay and Brenan 1973). Due to these unique features, Chlamydocola may turn out to be a good genus. Cola ficifolia, a palmately lobed simple-leaved species, also has stomata with thickened to papillate rims on the periclinal wall of the subsidiary cells and anisocytic stomata. Whether this species is closely related to those mentioned above (and those species which may or may not themselves be closely related) is unknown, but is worth investigating.
Paleobiogeography of Cola
The presence of Cola amharaensis in Ethiopia is interesting not only due to the absence of the genus in Ethiopia today, but also due to the fossil’s close morphological similarity with extant species found in the Guineo-Congolian forest region (West and Central Africa) as opposed to living species that are found in closer geographic proximity in Kenya, Somalia, and Sudan (Lebrun and Stork 2003; Fig. 1). At least two other fossil genera from the Guang River flora share this pattern of absence from Ethiopia today, and greater similarity to Guineo-Congolian species than to extant East African taxa. These include a fossil leaflet of the climbing calamoid palm Eremospatha (G. Mann & H. Wendl.) H. Wendl. (Pan et al. 2006), a genus restricted to West and Central Africa today, and several fossil leaflet specimens of Cynometra (Leguminosae) that are most similar to two Central African species (Pan 2007). In addition, fossil taxa with closer affinity to extant Guineo-Congolian forest elements (than to East African tropical forests) are also present in a Late Oligocene palynoflora from northeastern Kenya (Mansonia altissima type, Petersianthus type, and Triplochiton type; Vincens et al. 2006). These “exotic” taxa, as they are referred to in Vincens et al. (2006), and those from Ethiopia document a greater geographic distribution for some tropical moist forest elements in the Paleogene than their extant relatives, and indicate that extant species within these genera in eastern Africa may be more distantly related to these fossils than would otherwise be expected.
References
Alverson WS, Whitlock BA, Nyffeler R, Bayer C, Baum DA (1999) Phylogeny of the core Malvales: evidence from ndhF sequence data. Am J Bot 86:1474–1486
Bayer C, Kubitzki K (2003) Malvaceae. In: Kubitzki K, Bayer C (eds) The families and genera of vascular plants. Springer, Berlin, pp 225–310
Bodard M (1962) Contributions a l’étude systématique du genre Cola en Afrique Occidentale. Annales de Faculté des Sciences de l’Université de Dakar 7:1–187
Brenan JPM (1978) A new species of Cola (Sterculiaceae) from East Africa. Kew Bull 33:283–286
Cande SC, Kent DV (1995) Revised calibration of the geomagnetic polarity time scale for the Late Cretaceous and Cenozoic. Journal of Geophysical Research 100:6093–6095
Cheek M (2002) A new species of Cola (Sterculiaceae) from the Usambara Mountains, Tanzania. Kew Bull 57:417–422
Cheek M, Frimodt-Møller C (1998) The genus Octolobus (Sterculiaceae) new to East Africa. Kew Bull 53:682
Cheek M, Cable S, Hepper FN, Ndam N, Watts J (1996) Mapping plant diversity on Mount Cameroon. In: van der Maesen LJG, van der Burgt XM, de Rooy JM (eds) The Biodiversity of African Plants: Proceedings XIV AETFAT Congress 22–27 August 1994. Kluwer Academic Publishers, Wageningen, pp 110–120
Coates Palgrave K (1977) Trees of Southern Africa. C. Struik Publishers, Cape Town
Dilcher DL (1974) Approaches to the identification of angiosperm leaf remains. The Bot Rev 40:1–157
Ellis B, Daly DC, Hickey LJ, Johnson KR, Mitchell JD, Wilf P, Wing SL (2009) Manual of leaf architecture. Cornell University Press, Ithaca
Friis I (1992) Forests and forest trees of northeast tropical Africa. Royal Botanic Gardens, Kew
García Massini JL, Jacobs BF, Pan AD, Tabor N, Kappelman J (2006) The occurrence of the fern Acrostichum in Oligocene volcanic strata of the northwestern Ethiopian plateau. International Journal of Plant Sciences 167:909–918
Gehrig M (1938) Beiträge zur Pharmakognosie der Malvales. Anatomie des Laubblattes. Thesis, Base
Gentry AH (1988) Changes in plant community diversity and floristic composition on environmental and geographic gradients. Ann Mo Bot Gard 75:1–34
Germain R (1963) Flora Du Congo & du Rwanda et du Burundi. Spermatophytes 10(88): Sterculiaceae. Jardin botanique national de Belgique, Brussels
Hallé N (1961) Flore du Gabon, volume 2: Sterculiacées. Muséum National d’Histoire Naturelle, Paris
Hamilton A (1968) Some plant fossils from Bukwa. Ug J 32:157–164
Hamilton AC (1976) The significance of pattern of distribution. Palaeoecol Afr Surround Isl 9:63–97
Holmgren M, Poorter L, Siepel A, Bongers F, Buitelaar M, Chatelain C, Gautier L, Hawthorne WD, Helmink ATF, Jongkind CCH, Os-Breijer HJ, Wieringa JJ, van Zoest AR (2004) Ecological profiles of rare and endemic species. In: Poorter L, Bongers F, Kouamé FN, Hawthorne WD (eds) Biodiversity of West African Forests: an ecological atlas of woody plant species. CABI Publishing, Oxford, pp 101–389
Inamdar JA, Balakrishna Baht R, Ramana Rao TV (1983) Structure, ontogeny, classification, and taxonomic significance of trichomes in Malvales. K J Bot 26:151–160
Jacobs BF, Winkler DA (1992) Taphonomy of a middle Miocene autochthonous forest assemblage, Ngorora Formation, central Kenya. Palaeogeogr Palaeoclimatol Palaeoecol 99:31–40
Jacobs BF, Tabor N, Feseha M, Pan A, Kappelman J, Rasmussen T, Sanders W, Wiemann M, Crabaugh J, Garcia Massini JL (2005) Oligocene-age (32.7–27.5 Ma) terrestrial strata of northwestern Ethiopia: lithology, paleontology, and paleoclimate. Palaeontologia Electronica 8:1–19. http://palaeoelectronica.org/2005_1/jacobs25/issue1_05.htm
Judd WS, Manchester SR (1997) Circumscription of Malvaceae (Malvales) as determined by a preliminary cladistic analysis of morphological, anatomical, palynological, and chemical characters. Brittonia 49:384–405
Judd WS, Campbell CS, Kellogg EA, Stevens PF, Donoghue MJ (2008) Plant systematics: a phylogenetic approach, 3rd edn. Sinauer Associates Inc, Sunderland
Kappelman J, Rasmussen DT, Sanders WJ, Feseha M, Bown T, Copeland P, Crabaugh J, Fleagle J, Glantz M, Gordon A, Jacobs B, Maga M, Muldoon K, Pan A, Pyne L, Richmond B, Ryan T, Seiffert ER, Sen S, Todd L, Wiemann MC, Winkler A (2003) Oligocene mammals from Ethiopia and faunal exchange between Afro-Arabia and Eurasia. Nature 426:549–552
Keay RWJ, Brenan JPM (1973) Cola. In: Keay RWJ (ed) Flora of West Tropical Africa, volume 1, part 2, 2nd edn. The Whitefriare Press Ltd., London, pp 321–332
Koeniguer JC (1973) Les bois hétéroxylés de l’oasis de Kirdimi (Tchad). C.R. 96e Congr. Nat. Soc. Sav., Toulouse, 1971. Paris Sci 5:191–214
Lebrun J-P, Stork AL (2003) Tropical African flowering plants: ecology and distributions, volume 1 Annonaceae-Balanitaceae. Conservatoire et Jardin botanique de la ville de Genève, Switzerland
Lemoigne Y (1978) Flores tertiaires de la haute vallée de l’Omo (Ethiopia) Palaeontogr. Abt B Palaeophytol 165:89–157
Linder HP (2001) Plant diversity and endemism in sub-Saharan tropical Africa. J Biogeogr 28:169–182
Mabberley DJ (1997) The plant book: a portable dictionary of vascular plants, 2nd edn. Cambridge University Press, Cambridge
Mack GH, James WC, Monger HC (1993) Classification of paleosols. Geol Soc Am Bull 105:129–136
MacLatchy L, Deino A, Kingston J (2006) An updated chronology for the Early Miocene of NE Uganda. J Vertebr Paleontol 26(Supplement to 3):93A, abstract
Menzel P (1920) Über Pflanzenreste aus Basalttuffen des Kamerungebietes. Beiträge zur Geologischen Erforschung der Deutschen Schutzgebiete 18:17–32
Metcalfe CR, Chalk L (1950) Anatomy of the dicotyledons, volume I. Oxford University Press, London
Mohr PA (1971) The geology of Ethiopia. Addis Ababa University Press, Addis Ababa
Monteillet J, Lappartient J-R (1981) Fruits et graines du Cretace superieur des carriers de Paki (Senegal). Rev Palaeobot Palynol 34:331–344
Nkongmeneck B-A (1985) Un nouveau Cola (Sterculiaceae) du Cameroun. Bull. Mus. natn. Hist. nat. Paris, 4e sér., 7, section B. Adansonia 3:337–339
Pan AD (2007) The Late Oligocene (28–27 Ma) Guang River flora from the northwestern plateau of Ethiopia. Dissertation, Southern Methodist University, Dallas
Pan AD, Jacobs BF, Dransfield J, Baker WJ (2006) The fossil history of palms (Arecaceae) in Africa and new records from the Late Oligocene (28–27 Mya) of north-western Ethiopia. Bot J Linn Soc 151:69–81
Phillips O, Miller JS (2002) Global patterns of plant diversity: Alwyn H. Gentry’s forest transect data set. Missouri Botanical Garden, St. Louis
Sanders WJ, Kappelman J, Rasmussen DT (2004) New large-bodied mammals from the late Oligocene site of Chilga, Ethiopia. Acta Palaeontologica Polonica 49:365–392
Verdoorn IC (1981) The genus Cola in southern Africa. Bothalia 13:277–279
Vincens A, Tiercelin J-J, Buchet G (2006) New Oligocene-early Miocene microflora from the southwestern Turkana Basin, palaeoenvironmental implications in the northern Kenya Rift. Palaeogeography Palaeoclimatology Palaeoecology 239:470–486
Vollesen K (1995) Sterculiaceae. In: Edwards S, Tadesse M, Hedberg I (eds) Flora of Ethiopia and Eritrea, volume 2, part 2: Canellaceae to Euphorbiaceae. The National Herbarium and Uppsala University, Addis Ababa and Uppsala, pp 165–185
White F, Dowsett-Lemaire F, Chapman JD (2001) Evergreen forest flora of Malawi. Royal Botanic Gardens, Kew
Wilkie P, Clark A, Pennington RT, Cheek M, Bayer C, Wilcock CC (2006) Phylogenetic relationships within the subfamily Sterculioideae (Malvaceae/Sterculiaceae-Sterculieae) using the chloroplast gene ndhF. Syst Bot 31:160–170
Yemane K, Robert C, Bonnefille R (1987) Pollen and clay mineral assemblages of a late Miocene lacustrine sequence from the northwestern Ethiopian highlands. Palaeogeogr Palaeoclimatol Palaeoecol 60:123–141
Acknowledgment
We would like to thank the Authority for Research and Conservation of Cultural Heritage, the Ministry of Culture and Tourism, Ethiopia, and especially Ato Jara for permission to conduct our continuing research in northwestern Ethiopia, and the Director Mamitu Yilga and staff of the National Museum, Addis Ababa, and the Gondar ARCCH and Chilga Ministry of Culture and Sports Affairs for logistical support. We thank the Missouri Botanical Garden, the Royal Botanic Gardens at Kew, and the collectors of the herbaria specimens examined for assistance and access to their collections. We are grateful to the National Museums of Kenya, the Baringo Paleontological Research Project, the East African Herbarium, and Christine Kabuye for their collaborative support of work in Kenya. This project was funded by grants from the National Science Foundation (EAR-0001259, EAR-0240251, and EAR-0617306), the National Geographic Society, and the Dallas Paleontological Society. Tillehun Selassie, Misege Birara, Habtewold Habtemichael, Mesfin Mekonnen, and Drs. Ambachew Kebede and Aklilou Asfaw provided valuable field assistance. We thank Dr. Martin Cheek for generously sharing information about Cola and other sterculioids, for providing advice, and for supplying cuticle specimens from the Royal Botanic Gardens at Kew. We are grateful to Dr. Thomas Denk for images of the Cameroon sterculioid fossils housed at the Swedish Museum of Natural History. We also appreciatively acknowledge help from Yohannes Desta, Yeshiwass Sitotaw, Gebremeskel Ayele, Elias Addissu, and Teshome Yohannes at Chilga and laboratory assistance from Kathryn Larson.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Leaf and epidermal characteristics and states
-
1.
Leaf type: (0) simple, (1) palmately compound, (2) pinnately compound
-
2.
Leaf(let) shape: (0) elliptic, (1) obovate, (2) ovate, (3) oblong, (4) special (needles, awls, etc.)
-
3.
Leaf(let) attachment: (0) petiolate [petiolulate for compound-leaved species], (1) subsessile/sessile
-
4.
Leaf(let) texture: (0) chartaceous (1) semicoriaceous, (2) coriaceous
-
5.
Leaf(let) margin type: (0) entire, (1) lobed, (2) toothed
-
6.
Leaf(let) base angle: (0) acute, (1) obtuse, (2) wide obtuse
-
7.
Leaf(let) base shape: (0) cuneate/concave/decurrent, (1) convex/rounded, (2) truncate, (3) subcordate/cordate/lobate
-
8.
Leaf(let) apex morphology: (0) acute, (1) subacuminate, (2) acuminate, (3) obtuse/rounded/slightly emarginate, (4) deltate/cuspidate, (5) emarginate
-
9.
Primary venation: (0) pinnate, (1) tri-nerved at the base, (2) palmately veined
-
10.
Number of secondary vein pairs: (0) 0–5; (1) 6–10; (2) 11–15; (3) >16
-
11.
Secondary vein categories: (0) brochidodromous, (1) eucamptodromous, (2) festooned brochidodromous, (3) cladodromous, (4) reticulodromous, (5) toothed-craspedodromous, (6) toothed-semicraspedodromous, (7) toothed-festooned semicraspedodromous
-
12.
Tertiary venation: (0) random reticulate, (1) opposite percurrent, (2) alternate percurrent, (3) mixed opposite/alternate percurrent
-
13.
Abaxial epidermal cell shape: (0) isodiameteric, (1) rectangular
-
14.
Abaxial cell size: indicated by length (first set of numbers) and width ranges, rounded to the nearest 0.5 µm
-
15.
Abaxial epidermal cell arrangement: (0) random (a combination of any of the following cellular arrangements), (1) nonrandom–tetragonal, (2) nonrandom–pentagonal, (3) nonrandom–hexagonal, (4) nonrandom–polyagonal (>6-sided cells), (5) nonrandom–linear
-
16.
Abaxial anticlinal cell wall pattern: (0) straight, (1) rounded, (2) undulate
-
17.
Abaxial anticlinal cell wall shape of undulation: (0) U, (1) V, (2) Ω
-
18.
Abaxial epidermal cell surface ornamentation: (0) absent, (1) stippled, (2) striate
-
19.
Abaxial stoma: (0) absent, (1) present
-
20.
Abaxial stomatal complex type: (0) polycytic types (anomocyptic, cyclocytic), (1) anisocytic, (2) paracytic types (paracytic, brachyparacytic, amphibrachyparacytic, hemiparacytic), (3) tetracytic types (staurocytic, anomotetracytic, paratetracytic, brachyparatetracytic)
-
21.
Abaxial stomatal complex chains or clusters: (0) absent, (1) present
-
22.
Abaxial stomatal ornamentation: (0) absent, (1) striate, (2) papillate, (3) thickened areas on periclinal wall
-
23.
Abaxial guard cells: (0) level, (1) sunken, (2) raised
-
24.
Number of abaxial trichomes types: (0) glabrous/absent (no trichomes present), (1) one, (2) two, (3) three, (4) four, (5) five or more
-
25.
Abaxial hair types present: (0) unicellular simple, (1) unicellular glandular, (2) multicellular glandular, (3) stellate/peltate (usually only the polygonal base is preserved),
-
26.
Abaxial trichome dominance: (0) glandular trichomes predominate [≥70% glandular], (1) no trichome type predominates (29–69%), (2) stellate/peltate trichomes predominate [≥70% stellate/peltate], (3) simple hairs predominate (≥70% simple)
-
27.
Abaxial glandular hair pairs: (0) absent, (1) present
-
28.
Adaxial epidermal cell shape: (0) isodiameteric, (1) rectangular
-
29.
Adaxial cell size: indicated by length (first set of numbers) and width ranges, rounded to the nearest 0.5 µm
-
30.
Adaxial epidermal cell arrangement: (0) random (a combination of any of the following cellular arrangements), (1) nonrandom–tetragonal, (2) nonrandom–pentagonal, (3) nonrandom–hexagonal, (4) nonrandom–polyagonal (>6-sided cells), (5) nonrandom–linear
-
31.
Adaxial anticlinal cell wall pattern: (0) straight, (1) rounded, (2) undulate
-
32.
Adaxial anticlinal cell wall shape of undulation: (0) U, (1) V, (2) Ω
-
33.
Adaxial epidermal cell surface ornamentation: (0) absent, (1) stippled, (2) striate
-
34.
Adaxial stoma: (0) absent, (1) present
-
35.
Adaxial stomatal complex type: (0) polycytic types (anomocyptic, cyclocytic), (1) anisocytic, (2) paracytic types (paracytic, brachyparacytic, amphibrachyparacytic, hemiparacytic), (3) tetracytic types (staurocytic, anomotetracytic, paratetracytic, brachyparatetracytic)
-
36.
Abaxial stomatal complex chains or clusters: (0) absent, (1) present
-
37.
Adaxial stomatal ornamentation: (0) absent, (1) striate, (2) papillate, (3) thickened areas on periclinal wall
-
38.
Adaxial guard cells: (0) level, (1) sunken, (2) raised
-
39.
Number of adaxial trichomes types: (0) glabrous/absent (no trichomes present), (1) one, (2) two, (3) three, (4) four, (5) five or more
-
40.
Adaxial hair types present: (0) unicellar simple, (1) unicellular glandular, (2) multicellular glandular, (3) stellate/peltate (usually only the polygonal base is preserved)
-
41.
Adaxial trichome dominance: (0) glandular trichomes predominate (≥70% glandular), (1) no trichome type predominates (29–69%), (2) stellate/peltate trichomes predominate (≥70% stellate/peltate), (3) simple hairs predominate (≥70% simple)
-
42.
Adaxial glandular hair pairs: (0) absent, (1) present
Rights and permissions
About this article
Cite this article
Pan, A.D., Jacobs, B.F. The earliest record of the genus Cola (Malvaceae sensu lato: Sterculioideae) from the Late Oligocene (28–27 Ma) of Ethiopia and leaf characteristics within the genus. Plant Syst Evol 283, 247–262 (2009). https://doi.org/10.1007/s00606-009-0225-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-009-0225-1