Abstract
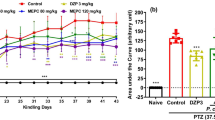
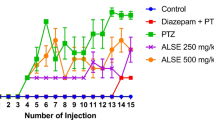
Alchemilla kiwuensis Engl. (Rosaceae) (A. kiwuensis) is an herbaceous plant traditionally used by Cameroonians to treat epilepsy and other central nervous system disorders. The present study evaluated the antiepileptogenic and antiepileptic effects of A. kiwuensis (40 mg/kg, 80 mg/kg) following Pentylenetetrazole (PTZ)-induced kindling as well as its sub-chronic toxicity. Following an initial i.p administration of a challenge dose (70 mg/kg), Wistar rats of both sexes received sub convulsive doses (35 mg/kg) of PTZ every other day, one hour after the oral gavage of animals with treatments, until two consecutive stage 4, in all animals of negative control. Seizure progression, latency, duration, and repetition were noted. Twenty-four hours later, animals were dissected to extract their hippocampi. The resulting homogenates were used to evaluate Malondialdehyde, reduced glutathione, catalase activity, GABA, GABA-Transaminase, glutamate, glutamate transporter 2, IL-1β and TGF-1 β. Sub-chronic toxicity was conducted according to OECD 407 guidelines. The lyophilisate of A. kiwuensis significantly increased the latency of seizure appearance, delayed seizure progression and decreased seizure repetition and duration. Biochemical analysis revealed that the lyophilisate significantly increased the catalase activity, reduced glutathione, GABA, glutamate transporter 2 and TGF-1B levels. The lyophilisate equally caused a significant decreased in the GABA-Transaminase activity, malondialdehyde, and IL-1 β levels. There was no noticeable sign of toxicity. A. kiwuensis possesses antiepileptic and antiepiletogenic effects by enhancing GABAergic neurotransmission and antioxidant properties, coupled to modulation of glutamatergic and neuroinflammatory pathways and is innocuous in a sub-chronic model. These justifies its local use for the treatment of epilepsy.










Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are adequately contained within the manuscript. Further information will be made available from the corresponding author on request from qualified investigators.
Change history
18 July 2023
The original article were updated due to removal of co-authors email addresses.
References
Linehan C, Benson A, Gunko A, Christensen J, Sun Y, Tomson T, Marson A, Forsgren L, Trinka E, Iliescu C, Althoehn J, Sonderup, Dreier JW, Sandu C, Leanca M, Rainer L, Kobulashvili T, Granbichler AC, Delanty N, Doherty C, Staines A, Shahwan A (2021) Exploring the prevalence and profile of epilepsy across Europe using a standard retrospective chart review: challenges and opportunities. Epilepsia 62:2651–2666. https://doi.org/10.1111/epi.17057
Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, Lagae L, Moshé SL, Peltola J, Perez ER, Scheffer I, Zuberi SM (2017) Operational classification of seizure types by the international league against epilepsy: position paper of the ILAE Commission for Classifcation and Terminology. Epilepsia 58:522–530. https://doi.org/10.1111/epi.13670
WHO (2019) Executive board 46th session provisional agenda item. 11 11:6
Jessica F-W, Scheffer IE, Robert S, Fisher (2018) The new definition and classification of seizures and epilepsy. Epilepsy Res 139:73–79. https://doi.org/10.1016/j.eplepsyres.2017.11.015
Mishra CB, Kumari S, Siraj F, Yadav R, Kumari S, Tiwari AK, Tiwari M (2018) The anti-epileptogenic and cognition enhancing effect of the novel 1-[4-(4-benzo [1,3] dioxol-5-ylmethyl-piperazin-1-yl)-phenyl]-3phenyl-urea (BPPU) in pentylenetetrazole induced chronic rat model of epilepsy. Biomed Pharmacother 105:470–480. https://doi.org/10.1016/j.biopha.2018.05.140
Rousseaux CG (2008) A review of glutamate receptor I: current understanding of their biology. J Toxicol Pathol 21:25–51. https://doi.org/10.1293/TOX.21.25
Sazhina TA, Sitovskaya DA, Zabrodskaya Y, Bazhanova ED (2020) Functional imbalance of glutamate-and GABAergic neuronal systems in the pathogenesis of focal drug-resistant epilepsy in humans. Bull Exp Biol Med 168:519–522. https://doi.org/10.1007/s10517-020-04747-3
Cho CH (2013) New mechanisms for glutamate hypothesis in epilepsy. Font Cell Neurosci 127:2. https://doi.org/10.3389/fncel.2013.00127
Nielsen EB, Suzdak PD, Andersen KE, Knutsen LJ, Sonnewald U, Braestrup C (1991) Characterisation of tiagabine (NO-328), a new potent and selective GABA uptake inhibitor. Eur J Pharmacol 196:257–266. https://doi.org/10.1016/0014-2999(91)90438-V
Greenfield JLJ (2013) Molecular mechanisms of antiseizure drug activity at GABAA receptor. Seizure 2013: 12p. doi: https://doi.org/10.1016/j.seizure.2013.04.015
Bjornsen LPBW (2016) Glutamate and GABA transporters in mesial temporal lobe epilepsy. Thesis for the degree of philosophiae Doctor. University of Oslo, Norway 2016: 42p. http://urn.nb.no/URN:NBN:no-53026
Treiman DM (2001) GABAergic mechanisms in Epilepsy. Epilepsia 42:8–12. https://doi.org/10.1046/j.1528-1157.2001.042suppl.3008.x
Fueta Y, Vasilets LA, Takeda K, Kawamura M, Schwarz W (2003) Down-regulation of GABA-transporter function by hippocampal translation products: its possible role in epilepsy. Neuroscience 118:371–378. https://doi.org/10.1016/s0306-4522(02)00924-7
Squire LR, Bloom FE, Spitzer NC, Du Lac S, Ghosh A, Berg D (2008) Fundamental neuroscience 3th Edition. Elsevier Academic press, Burlington
Pitkänen A, Lukasiuk K (2011) Mechanisms of epileptogenesis and potential treatment targets. Lancet Neurol 10:173–186. https://doi.org/10.1016/S1474-4422(10)70310-0
Reddy SD (2014) Clinical pharmacology of current antiepileptic drugs. IJPSN 1:2305–2319. https://doi.org/10.37285/ijpsn.2014.7.1.1
Akyuz E, Polat AK, Eroglu E, Kullu I, Angelopoulou E, Paudel YN (2020) Revisiting the role of neurotransmitters in epilepsy: an updated review. J Life Sci 265:60. https://doi.org/10.1016/j.lfs.2020.118826
Basulto JJJ, Martinez YJ, Cervantes MCR, Guerrero MEU, Velasco AIF, Zarate CB (2018) Interactions between epilepsy and plasticity. Pharmaceuticals 11:18. https://doi.org/10.3390/ph11010017
Reutt KKB, Czuczwar SJ (2020) Role of oxidative stress in epileptogenesis and potential implications for therapy. Pharmacol Rep 72:1218–1226. https://doi.org/10.1007/s43440-020-00143-w
Goddard VG, McIntyre DC, Leech CK (1969) A permanent change in brain functioning resulting from daily electrical stimulation. Exp Neurol 25:295–330. https://doi.org/10.1016/0014-4886(69)90128-9
Bertram E (2007) The relevance of kindling for human epilepsy. Epilepsia 48:67–74. https://doi.org/10.1111/j.1528-1167.2007.01068.x
Ngoupaye GT, Adassi MB, Foutsop FA, Yassi BF, Ngo bum E (2022) Pentylenetetrazole kindling-induced epilepsy rat models: insight on the severity state, a comparative study. IBRO neurosci Rep 13:164–176. https://doi.org/10.1016/j.ibneur.2022.08.003
Ngoupaye GT, Adassi MB, Foutsop FA, Noungoua MC, Yaya J, Kom DT, Ngo bum E (2021) Anticonvulsant effects and acute toxicity study of the aqueous lyophilised extract of four medicinal plants of Cameroon: Malvaviscus arboreus, Alchemilla kiwuensis and a mixture of Drymaria cordata and Markhamia lutea. Adv Tradit Med 22:177–191. https://doi.org/10.1007/s13596-020-00525-8
Adassi MA, Ngoupaye GT, Yassi FB, Foutsop AF, Kom TD, Ngo Bum E (2022) Revealing the most effective anticonvulsant part of Malvaviscus arboreus dill. Ex Cav. And its acute and sub-acute toxicity. J ethnopharmacol 303:21. https://doi.org/10.1016/j.jep.2022.115995
Singh T, Mishra A, Goel RK (2021) PTZ kindling model for epileptogeneis, refractory epilepsy, and associated comorbidities: relevance and reliability. Metab Brain Dis 36:1573–1590. https://doi.org/10.1007/s11011-021-00823-3
Erkeç ÖE, Arihan O (2015) Pentylenetetrazole Kindling epilepsy model. Epilepsia 2:6–12. https://doi.org/10.5505/epilepsi.2015.08108
Zhu X, Dong J, Shen K, Bai Y, Chao J, Yao H (2016) Neuronal nitric oxide synthase contributes to pentylenetetrazole-kindling induced hippocampal neurogenesis. Brain Res Bull 121:138–147. https://doi.org/10.1016/j.brainresbull.2016.01.010
De Souza A, Filho AJMC, Oliveira JVS, De Souza DAA, Lopes IS, De Carvalho MAJ, De Lima KV, Sousa FCF, Vasconcelos SMM, Macedo D, Fonteles MMD (2019) Prevention of Pentylenetetrazole-induced kindling in behavioural comorbidities in mice by levetiracetam combined with the GLP-1 agonist liraglutide: involvement of brain antioxidant and BDNF upregulating properties. Biomed Pharmacother 109:429–439. https://doi.org/10.1016/j.biopha.2018.10.066
Vilela LR, Lima IV, Kunsch EB, Pinto HPP, De Miranda AS, Vieira ELM, De Oliveira ACP, Moraes MFD, Teixeira AL, Moreira FA (2017) Anticonvulsant effects of cannabidiol in the pentylenetetrazole model: pharmacological mechanisms, electroencephalographic profile, and brain cytokine levels. Epilepsy Behav 75:29–35. https://doi.org/10.1016/j.yebeh.2017.07.014
Anissian D, Ghasemi-Kasman M, Khalili-Fomeshi M, Akbari A, Hashemian M, Kazemi S, Moghadamnia AA (2018) Piperine-loaded chitosan-STPP nanoparticles reduce neuronal loss and astrocytes activation in chemical kindling models of epilepsy. Int J Bio Macromol 107:973–983. https://doi.org/10.1016/j.ijbiomac.2017.09.073
Rohani R, Aliaghaei A, Abdollahifar MA, Sadeghi Y, Zare L, Dehghan S, Heidari MH (2021) Long-term effects of hippocampal low-frequency stimulations on pro-inflammatory factors and astrocytes activity in kindled rays. Cell J 23:85–92. https://doi.org/10.22074/cellj.2021.7139
Romoli M, Mazzocchetti P, D’Alonzo R, Siliquini S, Rinaldi VE, Verrotti A, Calabresi O, Costa C (2019) Valproic acid and epilepsy: from molecular mechanism to clinical evidences. Curr Neuropharmacol 17:926–946. https://doi.org/10.2174/1570159X17666181227165722
Stienen M, Haghikia A, Dambach H, Thöne J, Wiemann M, Gold R, Chan A, Dermietzel R, Faustmann MP, Hinkerohe D, Prochnow N (2011) Anti-inflammatory effects of the anticonvulsant drug levetiracetam on electrophysiological properties of astroglia are mediated via TGF1β regulation. Br J Pharmacol 162:491–507. https://doi.org/10.1111/j.1476-5381.2010.01038.x
Mahdavi A, Naeini AA, Najafi M, Maracy M, Ghazvini (2020) Effect of levetiracetam drug on antioxidant and liver enzymes in the epileptic patients: case-control study. Afr Health Sci 20:984–990. https://doi.org/10.4314/ahs.v20i2.55
Hauser WA (1997) Incidence and prevalence. In: Engel Jr J, Pedly TA, editors. Epilepsy, a comprehensive textbook. Philadelphia: Lippincott-Raven Publishers 47–57. doi:https://doi.org/10.1001/jama.300.4.443-a
Kwan P, Brodie MJ (2003) Early identification of refractory epilepsy. N Engl J Med 342:314–319. https://doi.org/10.1056/NEJM200002033420503
Wahab A (2010) Difficulties in treatment and management of epilepsy and challenges in new drugs development. Pharmaceuticals 3:2090–2110. https://doi.org/10.3390/ph3072090
Beghi M, Cornaggia CM, Frigeni, Beghi E (2006) Learning disorders in epilepsy. Epilepsia 47:14–18. https://doi.org/10.1111/j.1528-1167.2006.00681.x
Viswanatha GL, Venkataranganna MV, Prasad NBL (2017) Ameliorative potential of Colebrookea oppositifolia methanolic root extract against experimental models of epilepsy: possible role of GABA mediated mechanism. Biomed Pharmacother 90:455–465. https://doi.org/10.1016/j.biopha.2017.03.078
Muazu J, Kaita A (2008) A review of traditional plants used in the treatment of epilepsy among the Hausa/Fulani tribes of the northern Nigeria. Afr J Tradt Complement Altern Med 5:387–390. https://doi.org/10.4314/ajtcam.v5i4.31294
Ekor M (2013) The growing use of herbal medicine: issues realting to adverse reactions and challenges in monitoring safety. Front Pharmacol 177:10. https://doi.org/10.3389/fphar.2013.00177
Akbulut S, Bayramoglu MM (2013) The trade and use of some medicinal and aromatic herbs in turkey. Ethno Med 7:67–77. https://doi.org/10.1080/09735070.2013.11886446
Kamtchueng MO, Baylan R, Mouokeu RS, Tume C, Banerjee C, Chawla SA, Oumar M, Kuiate JR (2017) Anti-inflammatory activity of methanol extracts and fraction from Alchemilla kiwuensis eng on LPS activated macrophages. IJPPR 9:473–481. https://doi.org/10.25258/phyto.v9i2.8117
Kurtul E, Eryilmaz M, Sarialtin S, Tekin M, Acikara ÖB, Coban T (2022) Bioactivities of Alchemilla mollis, Alchemilla persica and their active constituents. Braz J Pharm Sci 58:10. https://doi.org/10.1590/s2175-97902022e18373
Kamtchueng MO, Mouokeu RS, Tume C, Djafoua YM, Kuiate JR (2013) Evaluation of immunomodulatory activity of the methanol extracts of Alchemilla kiwuensis Eng. IJPPR:349–355
Karhagomba IB, Mirindi TA, Mushagalusa TB, Nabino VB, Koh K, Kim HS (2013) The cultivation of wild food and medicinal plants for improving community livelihood: the case of the Buhozi site, DR Congo. Nutr Res Pract 7:510–518. https://doi.org/10.4162/nrp.2013.7.6.510
Tadic V, Krgovic N, Zugic A (2020) Lady’s mantle (Alchemilla vulgaris L., Rosaceae): a review of traditional uses, phytochemical profile, and biological properties. tr. Nat Med Mater 40:66–74. https://doi.org/10.5937/leksir2040066T
Vlaisavljevic S, Jelaca S, Zengin G, Mimica-Dukic N, Berezni S, Miljic M, Stevanovic Z (2019) Alchemilla vulgaris agg (Lady’s mantle) from central Balkan: antioxidant, anticancer and enzyme inhibition properties. RSC adv 9:37474–37483. https://doi.org/10.1039/c9ra08231j
Plevkova J, Brozmanova M, Harsanyiova J, Sterusky M, Honetschlager J, Buday T (2020) Various aspects of sex and gender bias in biomedical research. Physiol Res 69:367–378. https://doi.org/10.33549/physiolres.934593
Miller LR, Marks C, Becker JB, Hurn PD, Chen WJ, Woodruff T, McCarthy MM, Sohrabji F, Schiebinger L, Wetherington CL, Makris S (2017) Considering sex as a biological variable in preclinical research. FASEB J 31:29–34. https://doi.org/10.1096/fj.201600781R
Jefferys JGR (2003) Models and mechanisms of experimental epilepsies. Epilepsia 44:44–50
Kebriaeezadeh A, Emami S, Ebrahimi M, Sharifzadeh M, Khorosani R, Shafiee A (2008) Anticonvulsant and antiepileptogenic effects of azolylchromanones on lithium-pilocarpine induced seizures and pentylenetetrazole-kindling model of epilepsy. Biomed Pharmacother 62:208–211
Ngoupaye GT, Pahaye DB, Ngondi J, Moto FCO, Ngo Bum E (2017) Gladiolus dalenii lyophilisate reverses scopolamine-induced amnesia and reduces oxidative stress in rat brain. Biomed Pharmacother 91:350–357. https://doi.org/10.1016/j.biopha.2017.04.061
Ngoupaye GT, Kom TD, Adassi MB, Yaya J, Noungoua CM, Foutsop AF, Ngo Bum E (2020) Antiamnesic effect of aqueous lyophilisate of Drymaria cordata on scopolamine-induced amnesia and oxidative stress in mice. Invest Med Chem Pharmacol 3:45. https://doi.org/10.1016/j.biopha.2020.110603
Dimo T, Tsala DE, Dzeufiet DPD, Penlap BV, Njifutie N (2006) Effects of Alafia multiflora Stapf on lipid peroxidation antioxydant enzyme statuts in carbon tetrachloride-treated rats. Pharmacologyonline 2:76–89
Moto FCO, Arsa’a A, Ngoupaye TG, Taiwe GS, Njapdounke JSK, Kandeda AK, Nkantchoua GCN, Omam OJP, Pale S, Kouemou NE, Ayissi MER, Pahaye DB, Ojong L, Mairara V, Ngo Bum E (2018) Anxiolytic and antiepileptic properties of the aqueous extract of Cissus quadrangularis (Vitaceae) in mice pilocarpine model of epilepsy. Front Pharmacol 9:10. https://doi.org/10.3389/fphar.2018.00751
Bainbridge Z, Tomlins K, Wellings K, Westb A (1996) Methods for assessing Quality characteristics of Non-Grains Starch Staples (Part 3. Laboratory methods). NRI, Chatham, UK
Chang C, Yang M, Wan H (2020) Estimation of the total flavonoid content in Propolis by two complimentary calorimetric methods. J Food Drug Anal 10:178–182. https://doi.org/10.38212/2224-6614.2748
Singh DK, Srivastava B, Sahu A (2004) Spectrophotometric determination of rauwolfia alkaloids: estimation of Reserpine in Pharmaceuticals. Anal Sci 20:571–573. https://doi.org/10.2116/analsci.20.571
OECD (2008) OECD guidelines for the testing of chemicals N°407: repeated dose 28. -day oral toxicity study in rodent
Suvarna K, Layton C, Bancroft (2019) Bancroft’s theory and practise of histological techniques 9th edition. Elsevier: 557p
Akyüz E (2020) A new view of the racing scoring system in the pentylenetetrazol epilepsy model. J Harran Univ Med Fac 17:306–310. https://doi.org/10.35440/hutfd.763232
Deeba F, Sanz-Leon P, Robinson PA (2020) Effects of physiological parameter evolution on the dynamics of tonic-clonic seizures. PLoS ONE 15:25. https://doi.org/10.1371/journal.pcbi.1006387
Bhardwaj M, Kumar A (2016) Neuroprotective effects of Lycopene against PTZ-induced kindling seizures in mice: possible behavioral, biochemical and mitochondrial dysfunction. Phytother Res 30:306–313. https://doi.org/10.1002/ptr.5533
Kumar A, Lalitha S, Mishra J (2013) Possible nitric oxide mechanism in the protective effect of hesperidin against petylenetetrazol (PTZ)-induced kindling and associated cognitive dysfunction in mice. Epilepsy Behav 29:103–111. https://doi.org/10.1016/j.yebeh.2013.06.007
Taiwe GS, Dabole B, Tchoya TB, Menanga JR, Dzeufiet PDD, De Waard M (2016) Anticonvulsant effects of iridoid glycosides fraction purified from Feretia apodanthera Del. (Rubiaceae) in experimental mice models of generalized tonic-clonic seizures. BMC Complement Altern Med 16:285–307. https://doi.org/10.1186/s12906-016-1269-8
Tran V, Hatalski CG, Yan X, Baram TZ (2011) Effects of blocking GABA degradation on corticotropin releasing hormone gene expression in selected brain region. Epilepsia 40:1190–1197. https://doi.org/10.1111/j.1528-1157.1999.tb00847.x
Kennedy AD, Pappan KL, Donti T, Delgado M, Shinawi M, Pearson TS, Lalani SR, Craigen WJ, Sutton VR, Evans AM, Sun Q, Emrick LT, Elsea SH (2019) 2-Pyrrolidinone and succinimide as clinical screening biomarkers for GABA-transaminase deficiency: ant seizure medications impact accurate diagnosis. Front Neurosci 13:12. https://doi.org/10.3389/fnins.2019.00394
Kaur N, Singh T, Kumar S, Goel RK (2017) Neurochemical evidence based suggested therapy for safe management of epileptogenesis. Epilepsy behave 72:8–16. https://doi.org/10.1016/j.yebeh.2017.4.004
Zhou Y, Danbolt NC (2014) Glutamate as a neurotransmitter in the healthy brain. J Neural Transm 121:799–817. https://doi.org/10.1007/s00702-014-1180-8
Mazhar F, Malhi SM, Simjee SU (2017) Comparative study in the effects of clinically used anticonvulsants on the oxidative stress biomarkers in pentylenetetrazole-induced kindling model of epileptogenesis in mice. J Basic Clin Physiol Pharmacol 28:31–42. https://doi.org/10.1515/jbcpp-2016-0034
Popa-Wagner A, Mitran S, Sivanesan S, Chang E, Buga AM (2013) ROS and brain diseases: the good, the bad and the ugly. Oxid. Med. Cell. Longev 2013: 14p. doi: https://doi.org/10.1155/2013/963520
Roganovic M, Pantovic S, Dizdarevic (2019) Role of the oxidative stress in the pathogenesis of epilepsy. Neurol Sci Neurophysiol 36:1–8. https://doi.org/10.5152/NSN.2019.11632
Fokoua AR, Ajayi AM, Ben-Azu B, Chouna R, Folarin O, Olopade J, Nkeng-Efouet AP, Aderibigbe AO, Umukoro S, Nguelefack TB (2021) The antioxidant and neuroprotective effects of the Psychotria camptopus Verd. Hook. (Rubiaceae) stem bark methanol extract contributes to its antiepileptogenic activity against pentylenetetrazol kindling in male Wistar rats. Metab Brain Dis 36:2015–2027. https://doi.org/10.1007/s11011-021-00825-1
Borowicz-Reutt KK, Czuczwar SJ (2020) Role of oxidative stress in epileptogenesis and potential implications for therapy. Pharmacol Rep 72:1218–1226. https://doi.org/10.1007/s43440-020-00143-w
Simpson DSA, Oliver PL (2020) ROS generation in microglia: understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants 9:27. https://doi.org/10.3390/antiox9080743
Ganguly U, Kaur U, Chakrabarti SS, Sharma P, Agrawal BK, Saso L, Chakrabarti S (2021) Oxidative stress, neuroinflammation, and NADPH oxidase: implication in the pathogenesis and treatment of Alzheimer’s disease. Oxid. Med. Cell. Longev 2021: 19p. https://doi.org/10.1155/2021/7086512
Thompson KK, Tsirka SE (2017) The diverse role of microglia in neurodegenerative aspect of central nervous system (CNS) autoimmunity. Int J Mol Sci 18:15. https://doi.org/10.3390/ijms18030504
Hiragi T, Ikegaya Y, Koyama (2018) Microglia after seizure and in epilepsy. Cell 7:12. https://doi.org/10.3390/cells7040026
Wolinski P, Ksiazek-Winiarek D, Glabinski A (2022) Cytokines and neurodegeneration in epileptogenesis. Brain Sci 12:17. https://doi.org/10.3390/brainsci12030380
Vitkovic L, Maeda S, Sternberg E (2001) Anti-inflammatory cytokines: expression and action in the brain. Neuroimmunomodulation 1:295–312. https://doi.org/10.1159/000059387
Kwon JY, Jeon M, Jung UJ, Kim DW, Moon GJ, Kim SR (2019) Perspective: therapeutic potentials of flavonoids as alternative medicines in epilepsy. ASN 10:778–790. https://doi.org/10.1080/01926230252824725
Wasowski C, Marder M (2012) Flavonoids as GABAA receptor ligands: the whole story? J Exp Pharmaco 4:9–24. https://doi.org/10.2147/JEP.S23105
Hanrahan JR, Chebib M, Johnston GA (2015) Interactions of flavonoids with ionotropic GABA receptors. Adv Pharmaco 72:189–200. https://doi.org/10.1016/bs.apha.2014.10.007
Sefil F, Kahraman I, Dokuyucu R, Gokce H, Ozturk A, Tutuk O, Aydin M, Ozkan U, Pinar N (2014) Ameliorating effects of quercentin on acute pentylenetetrazole induced seizures in rats. Int J Clin Exp 7:2471–2477 PMCID: PMC4211749
Moghbelinejad S, Alizadeh S, Mohammadi G, Khodabandehloo F, Rashvand Z, Najafipour R, Nassiri-asl M (2017) The effects of quercentin on the gene expression of the GABAA receptor α5 subunit gene in a mouse model of kainic acid-induced seizure. J Physiol Sci 67:339–343. https://doi.org/10.1007/s12576-016-0497-5
Impellizzeri D, Cordaro M, Campolo M, Gugliandolo E, Esposito E, Benedetto F, Cuzzocrea S, Navarra M (2016) Anti-inflammatory and antioxidant effects of flavonoid-rich fraction of bergamot juice (bje) in a mouse model of intestinal ischemia/perfusion injury. Front Pharmacol 7:7. https://doi.org/10.3389/fphar.2016.00203
Leyva-lopez N, Gutierrez-Grijalva EP, Ambriz-Perez DI, Heredia JB (2016) Flavonoids as cytokine modulators: a possible therapy for inflammation-related disease. Int J Mol Sci 17:15. https://doi.org/10.3390/ijms17060921
Nassiri-asl M, Moghbelinejad S, Abbasi E, Yonesi F, Haghighi MR, Lotfizadeh M, Bazahang P (2013à Effects of quercentin on oxidative stress and memory retrieval in kindled rats. Epilepsy Behav 28: 151–155. doi: https://doi.org/10.1016/j.yebeh.2013.04.019
Cho H, Jeon S, Lee M, Kang K, Kang H, Park E, Kim M, Hong S, Seo S (2020) Analysis of the factors influencing body weight variation in Hanwoo Steers using an automated weighing system. Animals 10:8. https://doi.org/10.3390/ani10081270
Ghasemi A, Jeddi S, Kashfi K (2021) The laboratory rat: age and body weight matter. J Exp Clin Med 20:1431–1445. https://doi.org/10.17179/excli2021-4072
Uhuo EN, Ogugua VN, Emeka G (2014) A comparative study of the effects of ethanol bark and methanol leaf extracts of Kigelia africana on some biochemical parameters in alloxan induced diabetic rats. Glob J biotechnol Biochem 9:30–34. https://doi.org/10.5829/idosi.gjbb.2014.9.8491
Alsaadi BH, Shoaa HA, Sabrin RM, Amal AE, Dina SE, Gamal AM (2018) Hepatoprotective activity of costus species (Koen. Ex. Retz.) Against paracetamol-induced liver inhury in mice. Afr J Tradit Complement Altern Med 15:35–34. https://doi.org/10.21010/ajtcamv15i2.5
Jones R, Manickam C, Ram DR, Kroll K, Hueber B, Woolley G, Shah SV, Smith S, Varner V, Reeves K (2021) Systemic and mucosal mobilisation of granulocytes subsets during lentiviral infection. Immunology 164:348–357. https://doi.org/10.1111/imm.13376
Margraft A, Lowell CA, Zarbock A (2022) Neutrophils in acute inflammation: current concepts and translational implications. Blood 139:2130–2144. https://doi.org/10.1182/blood.2021012295
Sieminska I, Poljanska E, Baran J (2021) Granulocytes and cells of granulocytes origin-the relevant players in colorectal cancer. Int J Mol Sci 22:14. https://doi.org/10.3390/ijms22073801
Lewis RW, Bilington R, Debryune E, Gamer A, Lang B, Carpanini F (2002) Recognition of adverse and nonadverse effects in toxicity studies. Toxicol pathol 30:66–74. https://doi.org/10.1080/01926230252824725
Acknowledgements
The authors acknowledge the Research Unit of Animal Physiology and Phytopharmacology (URPAP) who hosted the present work.
Funding
Partial financial support was received by the International brain research organisation [IBRO Early Career grant] and the international society for neurochemistry [ISN CAEN − 1B] grants.
Author information
Authors and Affiliations
Contributions
A.F.F. co-designed the work, and conducted the laboratory trials, data analysis, and manuscript writing as part of its PhD thesis. G.T. N. designed the work and supervised the experiments, data analysis, and manuscript writing. Material preparation and data collection were performed by A.F.F. assisted by M.B.A., F. B.Y., T. D. K., C.M.N., and A.P., and supervised by G.T.N. and G.A. The first draft of the manuscript was written by A.F.F. and G.T.N. and G. A. commented the previous version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interest to disclose.
Ethics approval and consent to participate
Treatment of animals was done following the guidelines of the Cameroon bioethics committee (reg N. FWA IRB00001954) and in accordance with NIH-Care and use of laboratory animals manual, all of which are in line with Directives 2010/63/EU for animal experiments. Briefly, efforts were made to minimise animal suffering and to reduce the number of animal used in the experiment.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original article were updated due to removal of co-authors email addresses.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Foutsop, A.F., Ateufack, G., Adassi, B.M. et al. The Aqueous Lyophilisate of Alchemilla Kiwuensis Engl. (Rosaceae) Displays Antiepileptogenic and Antiepileptic Effects on PTZ-induced Kindling in rats: Evidence of Modulation of Glutamatergic and GABAergic Pathways Coupled to Antioxidant Properties. Neurochem Res 48, 3228–3248 (2023). https://doi.org/10.1007/s11064-023-03982-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-03982-0