Summary
Chlorophytum delicatulum Osborne, Vollesen & Bjorå from Zambia is described, illustrated and placed within the context of recent taxonomic and phylogenetic work on Chlorophytum in Africa. This delicate herb has leaf bases furnished with conspicuous dark red setae resembling eyelashes. Its phylogenetic position, ecology, phytogeography and conservation status are discussed. Chlorophytum delicatulum is assessed as being of Least Concern using the categories and criteria of the IUCN Red List.
Similar content being viewed by others
Introduction
Chlorophytum Ker Gawl. (Asparagaceae: Agavoideae Anthericeae) is a genus of approximately 150 species found across the Old World Tropics (Stevens 2001). The genus is particularly diverse in eastern and southern central Africa, with 52 species occurring in the Flora of Tropical East Africa (FTEA) area (Nordal et al. 1997), 57 species in the Flora Zambesiaca (FZ) area (Kativu et al. 2008) and 52 species in the Flore d’Afrique Centrale (FAC) area (Meerts 2015). Molecular analyses of the species of Chlorophytum have shown that they can be divided into subgroups that correlate with morphological characters and number of chromosomes (Bjorå 2008; Bjorå et al. 2017).
Material of the species described here was first collected at Mutinondo Wilderness Area in Eastern Zambia in 2010, and subsequent collections were made in 2015, 2016 and 2019. An earlier collection from 1955 from Serenje, also in the same general part of Zambia, has been referred to the same taxon. When detailed studies were carried out at Kew, this material could not be matched with any known species. It is described here as Chlorophytum delicatulum Osborne, Vollesen & Bjorå.
Materials and Methods
Morphological studies
Specimens were collected and studied by S. Bidgood, L. Merrett, J. Osborne and K. Vollesen at Mutinondo and extensively photographed by L. Merrett. Live specimens were studied in situ at Mutinondo. Herbarium specimens were studied at Royal Botanic Gardens, Kew (K). SEM photographs of seeds from Bidgood et al. 8600 (K) were prepared by Aurelie Grall at Kew.
Conservation status
In order to assess the conservation status of this new species, georeferences were taken or estimated from each herbarium collection, and extent of occurrence was calculated from a minimum convex polygon using the online GeoCAT tool (Bachman et al. 2011). A provisional assessment of conservation status was obtained by applying the categories and criteria of the IUCN Red List (IUCN 2012).
Molecular studies
Sequences from 33 accessions were aligned using MUSCLE v3.8.425 (Edgar 2004) within Geneious Prime v2020.2.4 (Kearse et al. 2012), under default parameter settings. Two datasets were established for the final analyses: (1) ITS1 with 33 accessions; and (2) plastid DNA (rps16 and trnL-F intergenic spacer) with the same 33 accessions. The best-fit model of nucleotide substitution for the alignments was selected using jModelTest v2.1.10 (Guindon et al. 2003; Diego et al. 2012) under the Akaike Information Criterion (AIC). The data were analysed using Maximum Likelihood and Bayesian inference phylogenetic methods. Maximum Likelihood analyses were conducted using the Randomized Axelerated Maximum Likelihood (RAxML) v8.2.11 (Stamatakis 2014) as implemented in Geneious Prime. Rapid Bootstrapping and search for best-scoring ML tree algorithms were used, and bootstrap analyses were performed with 1000 replicates. For the Bayesian phylogenetic analyses, Bayesian inference was performed using MrBayes v3.2.7a (Huelsenbeck & Ronquist 2001; Ronquist & Huelsenbeck 2003). Analyses were started using a random starting tree and run for four million generations, sampling every 1000 generations. Two Markov runs were conducted with four chains per run. To check whether the Markov Chain had converged well before finishing the analysis, the standard deviation of split frequencies (SDSF) was monitored to be below 0.01. The 1000 first generations (25%) were discarded as burn-in. The remaining trees were used for calculation of posterior probabilities and building a 50% majority-rule consensus tree. The trees were displayed with iTOL (Letunic & Bork 2016) and Keynote v10.0, Apple Inc.
Taxonomic Treatment
Chlorophytum delicatulum Osborne, Vollesen & Bjorå sp. nov. Type: Zambia, Muchinga Province, Mpika Distr., Mutinondo Wilderness Area, Mayense Camp, 3 May 2016, Bidgood, Merrett & Vollesen 8600 (holotype K! K000569940; isotypes O!, UZL!).
http://www.ipni.org/urn:lsid:ipni.org:names:77297514-1
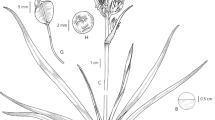
Erect, perennial herb, 15 – 55 cm tall, forming small tufts. Rhizome short, vertical, mostly covered by leaf bases and fibrous remains of old leaf bases. Roots wiry with fusiform distal tubers 0.8 – 1.5 × 0.7 – 1 cm. Cataphylls papery, 1 – 3 cm long, glabrous or apical part hispid to setose with dark red or white hairs. Leaves basal, distichous, linear, erect to spreading, 10 – 40 cm long, with distinct setose, sheathing bases. Leaf bases 2 – 7 × 0.1 – 0.25 cm, with papery margins and long dark red (occasionally white) setae 1 – 2 mm long along veins and margins. Leaf blades flat (with in-rolled margins or plicate when dry), 0.5 – 1.5 mm wide, nerves prominent, seven abaxially and four adaxially, hispid along the nerves with white hairs or a few red at the leaf tip. Inflorescences appearing with the leaves, 15 – 50 cm long, solitary or 2 (– 3) per plant, equal to or taller than the leaves. Peduncle terete or slightly flattened, longitudinally ridged, 10 – 45 cm tall, glabrous or sparsely hispid, without sterile bracts. Inflorescence racemose, 0.5 – 5 cm long, with one to four widely spaced nodes; basal internode (1 –) 1.5 – 4 cm long. Floral bracts uniformly dark reddish-purple or straw coloured with dark reddish-purple margins and apex, 3 – 6 mm long, ovate or broadly ovate, narrowing abruptly or gradually into a long caudate apex, with 3 – 5 veins, setose along veins and margins, setae 0.5 – 1 mm long, dark reddish-purple, turning whitish. Flowers open and star-shaped at anthesis, single or two per node; pedicels 2 – 5 mm long at anthesis, extending to 4 – 8 mm in fruit, articulated below the middle, glabrous. Tepals white with pale green 3 – 5-veined central stripes, glabrous; three outer ones linear-lanceolate, 6 – 9 × 1.5 – 2 mm, minutely papillose at apices, three inner ones broadly elliptic, 5 – 8 × 3 – 6 mm. Filaments white, glabrous, smooth, c. 1 mm long; anthers yellow, 2 – 3 mm long, papillose. Ovary sessile, ellipsoid, 1 – 2 × 0.75 – 1 mm; style 5 – 6.5 mm long, glabrous, bent outwards at anthesis; stigma truncate. Capsule partially covered by marcescent tepals when young, sub-globose, 3-locular, c. 5 × 4 mm, truncate to slightly retuse, with dense transverse ridges, style base persistent. Seeds black, irregularly folded, 1.7 – 2.5 × 1.5 – 1.7 mm; hilum forming a ridge 0.5 – 0.8 mm long, terminating in a minute acute projection (sometimes the hilum is not clearly visible, depending on how the seed is folded); testa glossy, echinulate-papillose. Figs 1 – 3.
Chlorophytum delicatulum. A habit; B distal portion of cataphyll; C proximal part of leaf; D – E leaf surfaces F floral bract; G floral bract, flattened; H flower, outside; J flower, inside; K anther and (right) enlarged surface of anther; L capsule; M open capsule; N seeds. A, F, G, M & N from Bidgood et al 8600; B – E from Merrett 2449; H, J, K & L from photos taken by L. Merrett. drawn by andrew brown.
recognition. Chlorophytum delicatulum is easily recognised by the combination of wiry roots with distal tubers, grass-like linear hairy leaves, racemose inflorescence, conspicuous dark red setae (resembling eyelashes) on cataphylls and basal part of the leaf margins, and bracts with long dark reddish-purple setae on veins and margins. It resembles narrow-leaved forms of C. galpinii (Baker) Kativu var. galpinii, which differ by almost always having a paniculate inflorescence, longer glabrous or very finely ciliate bracts (6 – 15 mm long, 3 – 6 mm long in C. delicatulum), and generally larger flowers (tepals 10 – 14 mm long, 5 – 9 mm long in C. delicatulum). Chlorophytum delicatulum is also similar to narrow-leaved forms of C. rubribracteatum (De Wild.) Kativu which typically have a reddish-purple coloration on cataphylls, leaf bases, bracts and tepal apices, and paniculate inflorescences. Some forms of C. sphagnicolum Meerts from NW Zambia with racemose inflorescences resemble the new species but differ in having generally broader leaves (1 – 2.5 (– 3) mm wide, 0.5 – 1.5 mm wide in C. delicatulum), and longer glabrous or very finely ciliate bracts ((5 –) 7 – 11 mm long, 3 – 6 mm long in C. delicatulum). A character matrix illustrates the morphological differences between C. delicatulum, C. rubribracteatum, C. sphagnicolum and the varieties of C. galpinii (Table 1). These three species also differ from C. delicatulum in their ecology. Chlorophytum delicatulum grows in pockets of sandy-gravelly or sandy-peaty soils in shallow, seasonally wet depressions on large granite outcrops or in rock crevices, C. galpinii and C. rubribracteatum are typically open woodland and grassland species, while C. sphagnicolum grows in ephemeral wet or swampy grassland pans on ironstone or dolomite soils.
One primary separating character for species groupings within Chlorophytum in recent floras (Nordal et al. 1997; Kativu et al. 2008; Meerts 2015) is the number of flowers per bract (either single or two to several per bract). Chlorophytum delicatulum blurs the difference between the groups insofar as the populations studied. For example, Bidgood 8231 (K) has some individuals with all flowers in an inflorescence being solitary, while others have at least some flowers paired. This variation appears not to be associated with the plants’ vigour.
distribution. Chlorophytum delicatulum is endemic to northern-central Zambia, in Mpika and Serenje Districts (Map 1). Due to the close proximity of this area to southern DR Congo (Katanga), it is quite possible that the species will eventually also be found there.
specimens examined. zambia. Central Province: Serenje Distr., Serenje, 23 Jan. 1955, Fanshawe 1839 (K!). Muchinga Province: Mpika Distr., Mutinondo Wilderness Area, Mayense Camp, 25 March 2010, Merrett 558 (K!) & 16 April 2015, Bidgood et al. 8231 (K!) & 3 May 2016, Bidgood et al. 8600 (K!, O!, UZL!) & 18 March 2019, Merrett 2449 (K!) & Choso Falls, 22 Feb. 2015, Osborne 1037 (UZL, K!).
habitat. At Mutinondo, Chlorophytum delicatulum grows in pockets of sandy-gravelly or sandy-peaty soils in shallow, seasonally wet depressions on large granite outcrops or in rock crevices. Dominant woody associates include Combretum molle R.Br. ex G.Don, Ficus ingens (Miq.) Miq., Landolphia parvifolia K.Schum., Lannea discolor (Sond.) Engl., Myrothamnus flabellifolius Welw., Ozoroa insignis Delile subsp. reticulata (Baker f.) J.B.Gillett, Tetradenia discolor Phillipson, Vitex mombassae Vatke and Xerophyta trichophylla (Baker) N.L.Menezes. Dominant perennial herbaceous species include Aloe mzimbana I.Verd. & Christian, A. zebrina Baker, Aeollanthus fruticosus Gürke, Coleochloa setifera (Ridl.) Gilly and Cyperus semitrifidus Schrad. A number of grasses are common associates and these include Diectomis fastigiata (Sw.) P.Beauv., Eragrostis welwitschii Rendle, Sporobolus festivus Hochst. ex A.Rich., Stereochlaena cameronii (Stapf) Pilg. and Trachypogon chevalieri (Stapf) Jacq.-Fél. Also present are a large number of annual herbs that include Chamaecrista mimosoides (L.) Greene, Commelina subulata Roth, Grona hirta (Guill. & Perr.) H.Ohashi & K.Ohashi, Exochaenium exiguum A.W.Hill (Sebaea minuta Paiva & I.Nogueira) and rare local endemics such as Crepidorhopalon mutinondoensis Eb.Fisch. & I.Darbysh. (Fischer et al. 2014), Euphorbia jubata L.C.Leach and Exochaenium alatum (Paiva & I.Nogueira) Kissling (Sebaea alata Paiva & I.Nogueira). The species has been collected at elevations from 1200 – 1450 m. Fig. 4.
conservation status. Chlorophytum delicatulum is known from only two sites in northern-central Zambia (Map 1). It has a very restricted distribution with an extent of occurrence of approximately 31 km2. The exact locality of the site at Serenje in Central Province, where this species was collected in 1955, is not known but is likely to be one of the hills that surround the town. There is extensive urban development and agricultural land conversion in this area, but the hills currently support natural or semi-natural vegetation and although the vegetation here is to some degree disturbed, Chlorophytum delicatulum may well still occur here. At the Mutinondo Wilderness Area in Muchinga Province, there have been five recent collections dating from 2010 – 2019. The species has been observed to be fairly frequent here and does not appear to be under threat. The almost bare granitic domes where it occurs do not contain any valuable minerals or gemstones and are of no agricultural use. Few of the woody species reach a size where they are of use for firewood and charcoal production. For these uses, there are better alternatives (e.g. Brachystegia) in nearby woodland habitats. Occasional trees which have died of natural causes may of course be collected for firewood, and some species (e.g. Aloe) are used medicinally. Grazing by wild hares, or possibly bushbuck, has been observed, but in no way represents a threat. Occasional burning, while killing all aerial parts, will not affect the tuberous root system. The Mutinondo Wilderness Area is currently managed for conservation and ecotourism, and Chlorophytum delicatulum is considered to be secure at this site. Because of its small stature and linear grass-like leaves the species is inconspicuous and likely to be overlooked. It may well be more widespread. Chlorophytum delicatulum is provisionally assessed as being of Least Concern (LC).
phenology. At Mutinondo and Serenje, flowering specimens have been observed or collected from the middle of the rainy season to the early dry season from late January to early May. Flowers are noted to close mid to late afternoon. Fruiting specimens have been collected from March to May.
When observed in the field, it is striking that plants of Chlorophytum delicatulum are never seen in groups or aggregations. Plants are always solitary, often occurring some considerable distance from the next plant. The significance of this population structure is not obvious. It could be an adaptation to herbivory (see below) or a strategy to counteract pests. This is a phenomenon also observed in several other species of Chlorophytum at Mutinondo, and here also in other plant families, e.g. in species of several genera in Apocynaceae subfam. Asclepiadoideae.
etymology. The specific epithet refers to the slender and delicate form of the plant.
Phylogenetic position
Phylogenetic analyses were performed to place the undescribed species in relation to other species of Chlorophytum. The analyses revealed the same general pattern as reported previously (Bjorå 2008; Bjorå et al. 2017). Thus, the general structure of the phylogenetic tree will not be discussed in detail here. Both in the nuclear (ITS, Fig. 5A) and concatenated chloroplast (Fig. 5B) analyses, C. delicatulum clusters together with species that were formerly included in genus Anthericum (Baker 1898; Kativu & Nordal 1993) and now informally referred to as the “Former African Anthericum group”. This group is highly supported (Fig. 5A & B, PP1/JK 100) in the molecular phylogeny and by morphology. The species are all characterised by wiry roots with distal tubers, most of them having distichous leaves, and all having shallow deltoid capsules that are transversely ridged and seeds that are irregularly folded. Species in this group are distributed from Ethiopia, southwards to the Cape, with more species occurring in southern Africa. Within this group, C. delicatulum resolves as sister to C. galpinii var. norlindhii (Weim.) Kativu, C. rubribracteatum, C. cameronii (Baker) Kativu and C. galpinii var. matabelense (Baker) Kativu (Fig. 5A, PP 0.94/JK 74). This clade is also seen in the chloroplast tree, but without robust support (Fig. 5B). The molecular analyses do not indicate a single sister species to C. delicatulum. Rather, it resolves as distinct from the “Former African Anthericum group” sister taxa included in the analyses (Fig. 5A & B). Since only a limited number of taxa from the “Former African Anthericum group” were included in the molecular study, further research is required to unravel the relationships among the closely related C. delicatulum, C. sphagnicolum and varieties of C. galpinii.
Two 50% majority rule consensus phylograms for species in the genus Chlorophytum from Bayesian analysis of A ITS dataset, B combined plastid DNA dataset. The Bayesian posterior probability values (PP) of at least 0.9 are stated in bold above branches and maximum parsimony jackknife support (JK) of at least 50 % are stated in italics below branches. Multiple accessions of the same taxon are numbered according to Table 2. Bars with names to the right represent informal morphological groups (following Bjorå 2008). Abbreviations: C = Chlorophytum, Con= Dem. Rep. Congo, Eth = Ethiopia, Gab = Gabon, Ken = Kenya, Mal= Malawi, Tan = Tanzania, Zam = Zambia, Zim = Zimbabwe.
References
Bachman, S., Moat, J., Hill, A. W., de la Torre, J. & Scott, B. (2011). Supporting Red List threat assessments with GeoCAT: geospatial conservation assessment tool. In: V. Smith & L. Penev (eds), e-Infrastructures for data publishing in biodiversity science. Zookeys 150: 117 – 126.
Baker, J. G. (1898). Anthericum. In: W. T. Thiselton-Dyer (ed.), Flora of Tropical Africa 7 (3): 477 – 493. Reeve, London.
Bjorå, C. S. (2008). Phylogeny, speciation and biogeography — a study of Crinum and Chlorophytum. PhD thesis, Faculty of Mathematics and Natural Sciences, University of Oslo.
____, Elden, M., Nordal, I., Brysting, A. K., Awas, T., Demissew, S. & Bendiksby, M. (2017). Speciation in the Genera Anthericum and Chlorophytum (Asparagaceae) in Ethiopia — a molecular phylogenetic approach. Phytotaxa 297: 139 – 156.
Diego, D., Guillermo, L. T., Ramón, D. & David, P. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9: 772.
Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic acids research 32: 1792 – 1797.
Fischer, E., Darbyshire, I. & Bingham, M. G. (2014). A new species of Crepidorhopalon (Linderniacaeae from the Mutinondo Wilderness, Zambia). Phytotaxa 181: 171 – 178.
Guindon, S., Gascuel, O. & Carvalho De Matos, C. (2003). A simple, fast, and accurate method to estimate large phylogenies by Maximum Likelihood. Syst. Biol. 52: 696 – 704.
Huelsenbeck, J. & Ronquist, F. (2001). MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754.
IUCN (2012). IUCN Red List categories and criteria, version 3.1, second edition. Gland and Cambridge.
Kativu, S. & Nordal, I. (1993). New combinations of African species in the genus Chlorophytum. Nord. J. Bot. 13: 59 – 65.
Kativu, S., Hoell, G., Bjorå, C. S. & Nordal, I. (2008). Anthericaceae. In: J. F. Timberlake (ed.), Flora Zambesiaca 13 (1): 34 – 89. Royal Botanic Gardens, Kew.
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., Buxton, S., Cooper, A., Markowitz, S., Duran, C., Thierer, T., Ashton, B., Meintjes, P. & Drummond, A. (2012). Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28: 1647 – 1649.
Letunic, I. & Bork, P. (2016). Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Research 44: W242 – W245.
Meerts, P. (2015). Anthericaceae. In: M. S. M. Sosef (ed.), Flore d’Afrique Centrale, nouvelle série. Jardin Botanique Meise, Meise.
Nordal, I., Kativu, S. & Poulsen, A. D. (1997). Anthericaceae. In: R. M. Polhill (ed.), Flora of tropical East Africa. A. A. Balkema, Rotterdam.
Ronquist, F. & Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572 – 1574.
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312 – 1313.
Stevens, P. F. (2001 onwards). Angiosperm Phylogeny Website. Version 12, July (2012, and continuously updated since).
Acknowledgements
The authors are grateful to Lari and Mike Merrett for supporting botanical research at Mutinondo Wilderness Area, to the University of Zambia (UNZA) and the Zambian Wildlife Authority (ZAWA) for providing Jo Osborne with Research Affiliation and Authority to Undertake Research in Zambia, and also to the Bentham-Moxon Trust for funding Jo Osborne and Florence Nyirenda to carry out fieldwork at Mutinondo. We are grateful to Kennedy Mapoule for his assistance and good company in the field and to Andrew Brown for his exquisite drawings. Thanks to Kine Hals Bødker for assisting with the molecular analyses and to Ann Ewankow for submitting sequences to GenBank. Our thanks are also due to Iain Darbyshire, Pierre Meerts and Inger Nordal for their advice and support during the preparation of the manuscript. We would also like to thank our reviewers for their helpful comments and suggestions.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Osborne, J., Vollesen, K., Bjorå, C.S. et al. Chlorophytum delicatulum (Asparagaceae), a newly described species from Zambia. Kew Bull 77, 675–684 (2022). https://doi.org/10.1007/s12225-022-10032-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12225-022-10032-5