Summary
Hartliella txitongensis Osborne & Eb.Fisch., a species new to science from Niassa Province in Northern Mozambique, is described and illustrated. Hartliella txitongensis is the only species of Hartliella known from outside the Upper Katanga region of the Democratic Republic of the Congo and the adjacent region of Zambia. The species is provisionally assessed as Critically Endangered under IUCN criterion B and its potential to be a metallophyte is highlighted. A key to the five species of Hartliella is provided.
Similar content being viewed by others
Introduction
The genus Hartliella Eb.Fisch. (Fischer 1992: 204) was erected to accommodate four suffruticose species formerly placed in a broadly defined Lindernia All. Species of Hartliella are characterised by a capitate inflorescence, almost coriaceous leaves, a woody rhizome, abaxial stamens with a knob-like basal appendage (only weakly pronounced in H. cupricola Eb.Fisch.), and aulacospermous seeds. The seeds of Linderniaceae can be divided into three different types: bothrospermous, aulacospermous and non-alveolated. In bothrospermous seeds the surface is sculptured due to the pit-like ingrowth of the inner epidermis of the ovule integument, the endothelium, into the endosperm. This endosperm alveolation results in characteristic rounded pits on the seed surface. By fusion of several endosperm pits, longitudinal furrows are produced that are typical of the aulacospermous seed type. In the non-alveolated seeds, the surface of the endosperm shows 5 – 6 rib-like shallow longitudinal furrows but no deeply impressed pits or ditch-like furrows (Fischer 1992). According to Fischer et al. (2013), Lindernia s.str. has non-alveolated seeds while the clades with bothrospermous seeds comprise e.g. the genera Torenia L. (Linnaeus 1753: 619), and Craterostigma Hochst. (Hochstetter 1841: 668) in a broad sense. The clade with aulacospermous seeds consists e.g. of Crepidorhopalon Eb.Fisch. (Fischer 1989: 443), Hartliella, and Bampsia Lisowski & Mielcarek (1983: 377). Up to now, four species of Hartliella are known, most of them endemic to Upper Katanga in the Democratic Republic of the Congo with one species, H. capitata (Eb.Fisch.) Eb.Fisch. (1992: 211) also occurring in adjacent Zambia. Hartliella suffruticosa (Lisowski & Mielcarek) Eb.Fisch. has an elongated stem with 3 – 4 pairs of broadly obovate to almost orbicular leaves and a small, basal pair of cataphylls, the upper leaf pair surrounding a few-flowered, capitate inflorescence. It is found in miombo woodland and savanna on heavy metal-rich soil and is endemic to Upper Katanga. The closely related H. cupricola differs from H. suffruticosa in having 4 – 6 pairs of lanceolate leaves, a longer calyx-tube and an undivided upper lip of the corolla. The upper leaf pair is distant from the inflorescence. The species is only known from the type locality at Kahumbwe in Upper Katanga (Fischer 1992; Malaisse et al. 2016) on copper-rich soil. The two further species have dense capitate inflorescences with a much smaller pair of surrounding leaves. Apart from the cataphylls, there are usually only two pairs of large leaves. The main difference between H. capitata and H. bampsii (Eb.Fisch.) Eb.Fisch. (1992: 209) lies in the arrangement of these two leaf pairs: in H. capitata, the internode is strongly condensed, thus appearing as a rosette or a whorl of four leaves, while in H. bampsii the internode is elongated with the upper pair arranged in the middle of the stem. Hartliella capitata is found in Upper Katanga with one locality in adjacent Zambia, mainly in miombo woodland on probably metalliferous soil while H. bampsii is endemic to Upper Katanga in similar, open woodland habitats. For further differences see Table 1.
During field work in northwest Mozambique in May 2019, the first author, together with Aurélio Banze and Papin Mucaleque, collected a specimen of Hartliella, that, at first glance, seemed intermediate between H. bampsii and H. capitata. Close study revealed that it represents a new species that is described below.
Materials and Methods
Plant material
Dried plant material, consisting of five individual plants of Hartliella txitongensis, was studied and imaged in the Herbarium at Kew. Of the five plants, at the time of collection, only one was in flower and the remaining plants were in varying stages of developing fruit. To avoid excessive destructive sampling from the flowering specimen, only two flowers were soaked in water and dissected for examination under a stereo microscope. Fruit and seed measurements were taken from the single most mature fruit found.
Maps
The maps were constructed in ArcMap version 10.3 to show the single locality where Hartliella is known to occur, and the locations of the Txitonga Mountains and Niassa province in Mozambique. The Txitonga mountains polygon was drawn manually using Google EarthTM imagery of the mountains and based on advice from a local guide and Government Environmental Officer (Fiscal) for the Lupilichi area (R.O. Saide pers. comm. 2019). The Basemap imagery was added from ArcGIS online.
Conservation status
The conservation status of Hartliella txitongensis was provisionally assessed using the IUCN categories and criteria (IUCN 2012).
Taxonomic Treatment
Hartliella txitongensis Osborne & Eb.Fisch. sp. nov. Type: Mozambique: Niassa Province, Txitonga (Chitonga) Mountains, Osborne JO 1711 (holotype LMA!; isotypes K! K001346951, LMU!).
http://www.ipni.org/urn:lsid:ipni.org:names:77297515-1
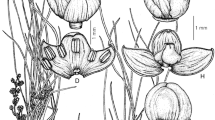
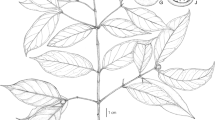
Perennial herb 4 – 10 cm tall, erect, growing from a woody rhizome. Stems glabrous, 4-angular and winged along the angles. Leaf pairs 2 (– 4). Leaves closely spaced at base, forming a rosette, sometimes with a more widely spaced internode above providing a pair of cauline leaves. Internodes 0 – 0.2 cm at base, 1.2 – 4 cm above. Leaves opposite, sessile, obovate to elliptic, 2 – 7.5 × 1 – 3.5 cm, base attenuate, margin entire or sometimes shallowly toothed towards apex, apex rounded to obtuse, venation palmate with mostly 3 or 5 main veins from the base, both leaf surfaces glabrous and minutely gland-dotted (visible at ×10 in dried material). Inflorescence terminal, capitate and many-flowered; bracts subtending the inflorescence lanceolate, up to 16 × 8 mm; bracts subtending individual flowers ranging from foliaceous lanceolate bracts similar to those that subtend the inflorescence to minute linear bracts 2 mm long; all bracts green, becoming dark red in fruit. Flowers sessile or shortly pedicellate, pedicels to 1.5 mm long. Calyx 10 – 14 mm long, glabrous, funnel-shaped, split to the base abaxially, 5 or 6 lobed, lobes unequal, 5 – 10 × 0.5 – 2 mm, lanceolate, margins entire or minutely serrulate. Corolla purple-blue with irregular dark and light markings at base of lower lip, glandular-puberulous, 17 – 18 mm long, tube 8 – 9 mm long, upper lip 5 × 3 mm, rounded at apex, undivided, lower lip 8 – 10 mm long, 3-lobed, lobes 3 mm long, central lobe broadly obovate, 4 mm wide, lateral lobes obovate 2 – 3 mm wide. Stamens 4, glabrous, filaments of upper pair 3 mm long, filaments of lower pair 5 mm long with a minute, bilobed appendage at base; anther thecae 0.5 mm long. Ovary globose, 1.5 mm, glabrous; style 10 mm long, glabrous; stigma two-lobed, lobes flattened, obtuse and minutely penicillate. Fruit a capsule, ovoid, 3 × 5 mm, shortly beaked at apex, beak c. 1 mm long, style persistent. Seeds (immature) 0.8 – 1 mm long, flat and oval or irregularly folded. Figs 1 & 2.
recognition. Hartliella txitongensis is similar to H. bampsii, but differs in the pairs of stem leaves being closely spaced with a short and condensed internode, thus forming a rosette at base (stem leaves are usually more distant in H. bampsii on a ± elongated stem), the leaves being obovate to elliptic, 2 – 7.5 × 1 – 3.5 cm (vs broadly obovate, 4 – 8 × 2.4 – 2.9 cm in H. bampsii), the slightly longer corolla tube, 8 – 9 mm long (vs 5 – 7 mm in H. bampsii), the undivided upper lip of the corolla (upper lip of corolla bifid in H. bampsii) and the bilobed appendage at the base of the abaxial filaments (simple globose in all other Hartliella species). It differs from Hartliella capitata in the elongated stem below the inflorescence and the shape of the leaves (these being broadly obovate to lanceolate, (3 –) 5 – 7.5 × (1.5 –) 3 – 6 cm in H. capitata) and the shorter corolla tube (12 mm long in H. capitata). A key to species of Hartliella is given below and differences are summarised in Table 1.
distribution. Africa: Mozambique. Known only from the type locality, probably endemic to the Txitonga Mountains. Map 1.
specimens examined. mozambique. Niassa Province, Txitonga (Chitonga) Mts, -11.82549, 35.05226, 19 May 2019, Osborne JO 1711 (holotype LMA!; isotypes K! K001346951, LMU!).
habitat. The vegetation on the foothills and slopes of the Txitonga Mountains is predominantly miombo woodland with narrow strips of moist gallery forest growing along deep stream gullies. At higher elevations, a more open montane savanna and montane grassland occurs.
At the type locality of Hartliella txitongensis, at 1515 m elevation, the woodland is open in places, grading into montane savanna grassland with rocky outcrops. The canopy is sparse and low at 3 m high. Woody species include Uapaca kirkiana Müll.Arg., Brachystegia spiciformis Benth., Parinari curatellifolia Planch. ex Benth., Protea spp., Erica mannii (Hook.f.) Beentje subsp. pallidiflora (Engl.) E.G.H.Oliv., Psorospermum febrifugum Spach and Morella pilulifera (Rendle) Killick. At higher elevations along the mountain ridge the low-growing shrubs Kotschya strigosa (Benth.) Dewit & P.A.Duvign and Cryptosepalum maraviense Oliv. are common. The montane grassland is mostly short, to c. 50 cm in height and rich in dwarf shrubs, herbs and geophytes. Dry-season fires occur regularly in the montane savannah grassland and the fire frequency is increased by the presence of gold miners (Osborne et al. 2019).
Hartliella txitongensis grows in full sun in a red loam soil at the type locality, occurring as scattered individuals in bare areas of leaf litter between grass clumps and also forming fairly dense patches in places. It appears to be highly localised.
conservation status. Hartliella txitongensis is highly range-restricted, currently known only from the type locality and probably endemic to the Txitonga Mountains of northern Mozambique. This locality is nominally a protected area (Lake Niassa Reserve) but the vegetation of miombo woodland, moist gallery forest, montane savanna and grassland is not effectively protected. The locality is particularly threatened by gold mining, which causes habitat loss locally, broad disruption to the hydrology and environmental damage such as areas with broken rocks, extremely eroded ruptures, sedimented rivers and mercury contamination. The frequency of uncontrolled wildfires is intensified due to the expansion and presence of gold miners which constitute both a current and future threat to the vegetation. However, fire may not be a threat to this species as it grows from a woody rhizome and these resprout after fires, and colonise vegetatively, providing some resilience to recurrent fires (Pausas et al. 2018). Since two of the four other species of Hartliella are known to occur on metal-rich soils, there is a possibility that H. txitongensis is a metallophyte occupying a restricted ecological niche. Using a precautionary approach based on the single known site for this species, the area of occupancy (AOO) is estimated to be less than 10 km2. With an extent of occurrence (EOO) of less than 100 km2, an area of occupancy (AOO) estimated to be less than 10 km2, a single known location and little-known ecological niche, in addition to current threats to the vegetation and an inferred continuing decline in its habitat, this species is provisionally assessed as Critically Endangered under IUCN criterion B: CR B1ab(iii) + 2ab(iii).
The Txitonga mountains have a unique biogeography for Mozambique and therefore a high biodiversity value. The site is to be recognised as an Important Plant Area for Mozambique (Osborne et al. in prep). In light of the biodiversity value of this area, conservation measures are urgently needed to protect the flora. Since there is already local management in the area through the Mining Associations, there is potential to put a conservation management plan in place.
phenology. The type was collected in mid-May when most of the population was in fruit (seeds immature). Phenology is estimated as: flowering in April – May, fruiting in May – June. The phenology is similar to that of many other herb taxa recorded during the fieldwork in the Txitonga mountains, flowering and fruiting during the period following the summer rains that occur between November and April (MAE 2005). Hartliella txitongensis appears to flower during the early part of the period following the rains.
etymology. Named after the Txitonga (= Chitonga) Mountains in order to highlight the conservation value of the area.
Key to the species of Hartliella
-
1. Stems elongated, plants 6 – 20 cm tall, with 3 – 6 decussate pairs of leaves (excluding cataphylls), inflorescence a few-flowered capitulum …………………………………………………….....................................................................…….2
-
1.' Stems condensed, plants 1 – 10 cm tall, with 2 (– 4) decussate pairs of leaves (excluding cataphylls), inflorescence a dense, multi-flowered capitulum ……………………………………………………………………3
-
2. Leaves broadly obovate, 1.5 – 2.5 × 1.2 – 1.6 cm, stem up to 6 – 11 cm tall, with 3 – 4 pairs of leaves, upper leaves surrounding the capitulum usually not considerably smaller than mid-stem leaves, calyx tube 1 mm long, upper lip of corolla bifid, Upper Katanga……… ……………………………………………………H. suffruticosa
-
2.' Leaves lanceolate, 3.5 – 4.2 × 0.7 – 0.95 cm, stem 14 – 20 cm tall, with 4 – 6 pairs of leaves, upper leaves surrounding the capitulum much smaller than mid-stem leaves, calyx tube 8 mm long, upper lip of corolla entire, Upper Katanga …………………………………………………………………………………....H. cupricola
-
3. Stem with strongly condensed internodes, plants 1 – 2 cm tall, with the leaves surrounding the dense capitulum like an involucrum, corolla tube 12 mm long, upper lip of corolla entire, Upper Katanga, NW Zambia………………………………………………………………………………………………………...H. capitata
-
3.' Stem less condensed, plants 2 – 10 cm tall, with at least one elongated internode below the dense capitulum, corolla tube either 8 – 9 or 5 – 7 mm long, upper lip entire or bifid…....4
-
4. Stem leaves forming a rosette at base, corolla tube 8 – 9 mm, upper lip of corolla undivided, appendage at the base of the abaxial filaments bilobed, Mozambique, Niassa ……………………………………….H. txitongensis
-
4.' Stem leaves not forming a rosette at base, usually more distant on a ± elongated stem, corolla tube 5 – 7 mm long, upper lip of corolla bifid, appendage at the base of the abaxial filaments simple-globose, Upper Katanga ………………………………………………………………………………………………………………...H. bampsii
Discussion
The study area
Niassa Province in north-west Mozambique is the most sparsely populated province in the country. It borders Tanzania to the north and Malawi and Lake Niassa to the west. Nineteen of Mozambique’s endemic plant taxa occur in Niassa Province (10 of these occur only in Niassa) in addition to 21 regional near-endemic taxa (Darbyshire et al. 2019) (Table 2). The discovery of additional endemics and near-endemics is highly likely as Niassa Province is under-explored botanically. The province has two protected areas, the huge Niassa National Reserve, also covering part of Cabo Delgado Province, and the Lake Niassa Reserve, a Ramsar site including both Lake Niassa and the adjacent coastal zone (Ramsar 2011). The Hartliella species described here was collected during botanical fieldwork to explore the mountains of the Lupilichi area of Lago district (Map 1), within the Lake Niassa Reserve. These mountains, in the northwest of Lago district, extend south from the southern end of the Kipengere Range in Tanzania and form part of the eastern escarpment of the East African rift. They are isolated from the other highland areas in Niassa province — the broad Lichinga plateau area and outlying Mt Mecula and Mt Yao. Mt Txitonga (Chitonga) is the highest peak in the range at c. 1848 m elevation. The mountain range is referred to here as the Txitonga Mountains.
Fieldwork
Fieldwork in the Txitonga Mountains was undertaken as part of the Mozambique Tropical Important Plant Areas (TIPAs) project, a collaboration between the Royal Botanic Gardens, Kew, Mozambique’s Agricultural Research Institute (IIAM) and Eduardo Mondlane University, with the aim of identifying and promoting the long-term conservation and sustainable management of Mozambique’s most important sites for plant diversity (Darbyshire et al. 2019). The Txitonga Mountains were selected as a target site for fieldwork because they are little-known botanically and likely to support a distinct biodiversity for Mozambique, closely allied to that of southwest Tanzania.
Fieldwork took place over eight days in May, working from a field camp in the foothills above Tulo Calanda village (Osborne et al. 2019). The aims of the fieldwork were to (i) document the vegetation; (ii) record current levels of protection and threats to the vegetation; (iii) document populations of endemic and near endemic target species; (iv) provide a preliminary species inventory. Throughout the fieldwork, plant specimens, with accompanying data and photographs, were collected for identification and as a tangible record of occurrence. The specimens were pressed in the field and dried over gas in the field camp. Additional tissue samples were collected into silica gel to facilitate molecular analysis. During the course of the fieldwork 149 plant specimens were collected and specimen identification is ongoing in the herbaria at Kew (K) and IIAM (LMA). In addition to Hartliella txitongensis, two other potentially new taxa were collected, a Streptocarpus Lindl. (Gesneriaceae) and a Bothriocline Oliv. ex Benth. (Asteraceae / Compositae), and further study is underway to confirm their status. It possible that other new taxa may be identified.
Potential to be a metallophyte
Whilst two of the four other species of Hartliella are known to occur on metal-rich soils, the soil composition at the type locality of H. txitongensis is not yet known. Since there is frequent gold mining in the Txitonga mountains, there is a high likelihood that other metals are present in the soil. Soil analysis and further ecological research at the type locality would be of great interest. If H. txitongensis is found to be a metallophyte, there is potential application of the newly described taxon in soil remediation (Ali et al. 2013; Awa & Hadibarata 2020).
References
Awa, S. H. & Hadibarata, T. (2020). Removal of Heavy Metals in Contaminated Soil by Phytoremediation Mechanism: a Review. Water, Air, & Soil Pollution 231: 47. https://doi.org/10.1007/s11270-020-4426-0
Ali, H., Khan, E. & Sajad, M. A. (2013). Phytoremediation of heavy metals — concepts and applications. Chemosphere 91 (7): 869 – 881. https://doi.org/10.1016/j.chemosphere.2013.01.075
Darbyshire, I., Timberlake, J., Osborne, J., Rokni, S., Matimele, H., Langa, C., Datizua, C., Sousa, C. de, Alves, T., Massingue, A., Hadj-Hammou, J., Dhanda, S, Shah, T. & Wursten, B. (2019). The endemic plants of Mozambique: diversity and conservation status. Phytokeys 136: 45 – 96. https://doi.org/10.3897/phytokeys.136.39020
Fischer, E. (1989). New species of Lindernia Allioni and Crepidorhopalon E. Fischer (Scrophulariaceae) from Zaire, Burundi and Tanzania. Bull. Jard. Bot. Natl. Belg. 59: 445 – 457.
____ (1992). Systematik der afrikanischen Lindernieae (Scrophulariaceae). Trop. Subtrop. Pflanzenwelt 81: 1 – 365.
____, Schäferhoff, B. & Müller, K. (2013). The phylogeny of Linderniaceae — The new genus Linderniella, and new combinations within Bonnaya, Craterostigma, Lindernia, Micranthemum, Torenia and Vandellia. Willdenowia 43: 209 – 238. https://doi.org/10.3372/wi.43.43201
Hochstetter, C. F. (1841). Nova genera plantarum Africae tum australis tum tropicae borealis: Craterostigma. Flora 24 (2): 668 – 670.
IUCN (2012). IUCN Red List categories and criteria, version 3.1, second edition. Gland and Cambridge.
Linnaeus, C. (1753). Species Plantarum, exhibentes plantas rite cognitas, ad genera relatas, cum differentiis specificis, nominibus trivialibus, synonymis selectis, locis natalibus secundum systema sexual digestas. Tomus II: 561 – 1200. Holmiae.
Lisowski, S. & Mielcarek, R. (1983). Un nouveau genre et deux nouvelles espèces de Scrophulariacées au Zaïre. Bull. Jard. Bot. Natl. Belg. 53: 377 – 381. https://doi.org/10.2307/3667798
MAE (Ministério de Administração Estatal) (2005). Perfil do Distrito de Lago, Província de Niassa. Perfis Distritais 2005. https://www.biofund.org.mz/wp-content/uploads/2018/12/Lago-perfil-distrital.pdf
Malaisse, F., Schaijes, M. & D’Outreligne, C. (2016). Copper-Cobalt Flora of Upper Katanga and Copperbelt. Les presses agronomiques de Gembloux.
Osborne, J., Datizua, C., Banze, A., Mamba, A., Mucaleque, P. & Rachide, T. (2019). Niassa Province – Lago District mountains and Njesi Plateau, May 2019. Mozambique TIPAs Fieldwork Report (unpublished).
____, Richards, S. & Darbyshire, I. (in prep.). Txitonga Mountains Important Plant Area. In: I. Darbyshire, S. Richards, J. Osborne, H. Matimele, C. Langa, C. Datizua, A. Massingue, S. Rokni, J. Williams, T. Alves & C. de Sousa, The Important Plant Areas of Mozambique. Royal Botanic Gardens, Kew.
Pausas, J. G., Lamont, B. B., Paula, S., Appezzato-da-Glória, B. & Fidelis, A. (2018). Unearthing belowground bud banks in fire-prone ecosystems. New Phytol. 217: 1435 – 1448. https://doi.org/10.1111/nph.14982
Ramsar (2011). Lake Niassa and its Coastal Zone (Lago Niassa e Zona Costeira). Ramsar Sites Information Service. https://rsis.ramsar.org/ris/1964
Acknowledgements
The authors would like to thank all those who supported the fieldwork in the Txitonga Mountains that has led to this publication. In particular, we would like to thank Camila de Sousa and Tereza Alves at the Instituto de Investigação Agrária de Moçambique (IIAM) and Iain Darbyshire at the Royal Botanic Gardens, Kew for their roles in leading the Mozambique Tropical Important Plant Areas (TIPAs) project. In Lago district, we are grateful to Sr Mbumba and Sr Nandja from WWF who provided advice on access into the mountains and Sr Amisse Arabe at the Secretaria do Posto Administrativo de Cóbué who provided advice on local government administration in the Lupilichi area. In Tulo Calanda mining village, we thank the Presidente Geral of the Mining Associations, Sr Calisto Pedro and the Chefe da Localidade de Lupilichi, Sr Calunga Ali, who provided permissions and advice. Especial thanks go to our IIAM colleagues in our field team, Aurélio Banze, Aristides Mamba and Tomé Rachide, to our local guides Sr Saide Omade Maoji and Sr Ricardo Omar Saide, Government Environmental Officers (Fiscais) for Lupilichi area, and to our drivers, Luis de Sousa Guidione from IIAM Centro Zonal Noroeste and Sansão Marcos Gribate from IIAM Centro Zonal Nordeste.
We are very grateful to The Jonathan and Jennifer Oppenheimer Foundation, and to Stephen and Margaret Lansdown for their generous support of the Mozambique Tropical Important Areas project that funded the fieldwork in Niassa Province.
The authors would also like to thank the anonymous reviewers for their helpful comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Osborne, J., Datizua, C., Mucaleque, P. et al. Hartliella txitongensis (Linderniaceae), a new species from Mozambique. Kew Bull 77, 665–673 (2022). https://doi.org/10.1007/s12225-022-10034-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12225-022-10034-3