Summary
A new genus, Lukea Cheek & Gosline (Annonaceae), is erected for two new species to science, Lukea quentinii Cheek & Gosline from Kaya Ribe, SE Kenya, and Lukea triciae Cheek & Gosline from the Udzungwa Mts, Tanzania. Lukea is characterised by a flattened circular bowl-shaped receptacle-calyx with a corolla of three free petals that give the buds and flowers a unique appearance in African Annonaceae. Both species are extremely rare shrubs of small surviving areas of lowland evergreen forest under threat of habitat degradation and destruction and are provisionally assessed as Critically Endangered and Endangered respectively using the IUCN 2012 standard. Both species are illustrated and mapped. Material of the two species had formerly been considered to be possibly Uvariopsis Engl. & Diels, and the genus Lukea is placed in the Uvariopsis clade of the Monodoreae (consisting of the African genera Uvariodendron (Engl. & Diels) R.E.Fries, Uvariopsis, Mischogyne Exell, Dennettia Baker f., and Monocyclanthus Keay). The clade is characterised by often conspicuous, finely reticulate quaternary nervation, incomplete or absent connective shields (in Annonaceae the connective shield is usually complete) and free petals (except in some Uvariopsis). Morphologically Lukea is distinct for its broad, turbinate, fleshy pedicel, a potential synapomorphy within Monodoreae. It appears closest morphologically to the West African monotypic Monocyclanthus, since it shares a trait unusual in the Annonaceae: the calyx in both genera forms a shallow bowl (calyx lobes are absent or vestigial), barely enclosing the base of the petals at anthesis, which persists, living and green, in the mature fruit. However, on recent molecular phylogenetic evidence, Lukea is sister to Mischogyne and the two split c. 20 million years BP, while Monocyclanthus is sister to Dennettia. The placement of Lukea within the Uvariopsis clade is discussed.
Similar content being viewed by others
Introduction
The Annonaceae is a pantropical family largely restricted to tropical rainforest habitats. The characteristic often showy flowers and fruit help make it widely collected and studied (Chatrou et al. 2012a). Annonaceae are well known for species producing edible fruits (paw paw, soursop, custard apple), and for the fragrant ylang-ylang (Cananga odorata (Lam.) Hook.f. & Thomson) used in perfumes. The Annonaceae is a basal angiosperm family in the order Magnoliales (Heywood et al. 2007). It comprises c. 2500 species and c. 111 genera of trees, shrubs and lianas (Chatrou et al. 2012b; Guo et al. 2017). Within the subfamily Annonoideae, the tribe Monodoreae is composed of 11 genera and 92 species restricted to Africa and Madagascar (Guo et al. 2017; Dagallier 2021).
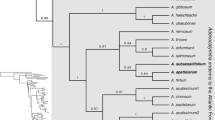
In December 1953, Semsei 1520 (EA, K) an herbarium specimen of Annonaceae was collected from Mtibwa Forest Reserve, Morogoro Distr., Tanzania. Sterile, it was initially tentatively identified as possibly a Polyalthia Blume, later as probably a Uvariodendron (Engl. & Diels) R.E.Fries (Monodoreae), although this was doubted by Verdcourt. More than 40 years later Luke collected Luke 4703 (EA, K) also sterile, from Kaya Ribe in the coastal forests of Kenya. Recollected in Sept. 1997 (Luke & Luke 4740 (K) with flower buds and fruit, the latter two specimens were identified as “Annonaceae Genus Nov.” by Vollesen at Kew in 1998 and set aside for description. A further collection of the taxon from Kaya Ribe with open flowers and fruit was made in Jan. 1999 (Luke 5700, K) (Figs 1 & 2). Luke and colleagues recollected the Kaya Ribe taxon in flower and fruit in May 2021 (Luke et al. s.n. EA). Two further collections were made of the Tanzanian taxon in 2003 and 2005, making flowers and fruit available for the first time (Luke et al. 9526, 11205, both K) (Figs 3 & 4). Independently of Vollesen, both species were provisionally identified by Luke as Uvariopsis Engl. & Diels. Vollesen was not able to research the material further, being required to complete the Flora of Tropical East Africa account for Acanthaceae before his retirement (Darbyshire et al. 2010). The material at K of his new genus was passed to Gosline in 2017 together with other unresolved East African Annonaceae. Two of these, Marshall 1567 and Cribb & Grey-Wilson 10082, were placed in Mischogyne Exell using molecular methods and prompted a revision of that Afrotropical genus (Gosline et al. 2019). The new genus identified by Vollesen was placed by Gosline in Monodoreae, an entirely African tribe, in the region of Uvariopsis while noting the similarity of venation with Mischogyne. These two genera are placed in a clade together with Uvariodendron and Monocyclanthus Keay (referred to here as the Uvariopsis clade) by Guo et al. (2017) and Gosline et al. (2019). The position remained uncertain until a molecular phylogenetic analysis of the whole Monodoreae tribe using a new set of nuclear markers (Couvreur et al. 2019) was undertaken by Dagallier (Dagallier 2021). Dagallier’s phylogenetic study was based on 315 nuclear genes sequenced for almost all (88 of the 92 described) species of the tribe. The results indicated that the sequenced specimens Luke 11205 and 9526 (respectively type and paratype specimens of Lukea triciae) indeed clustered in Monodoreae, and more specifically in the informal “Uvariopsis clade”. The Lukea specimens were recovered with maximum support as sister to Mischogyne, the two being sister to the rest of the Uvariopsis clade (Dagallier 2021). However, both Lukea and Mischogyne showed important genetic differentiation (long branches separating both genera which are dated as splitting from each other c. 20 million years BP (Dagallier 2021)), confirming the new genus status as first indicated by Vollesen. To date, no specimen of L. quentinii has been sequenced.
Lukea quentinii A habit, leafy, flowering stem, leaves showing adaxial surface; B habit, flowering node, showing abaxial surface of blade; C close-up of B; D hairs on abaxial midrib; E hairs on distal stem internode; F flower bud, lateral view; G flower bud, plan view; H flower with petal removed to show stamens and carpels; J petal, abaxial surface; K vestigial inner petal; L stamen, showing lateral pollen thecae and incomplete connective cap; M pistil, side view; N flower post anthesis/young fruits; P immature fruit with persistent calyx; Q mature fruit (dehydrated, showing constrictions); R seed, transverse section. A – E, G – N, R from Luke et al. 5700; F, P, Q from Luke et al. 4740. drawn by andrew brown.
Lukea triciae A habit, leafy, flowering stem, leaves showing adaxial and (most distal leaf) abaxial surfaces; B detail of node showing abaxial leaf surface; C flower bud, side view; D flower bud, plan view; E flower bud, view from pedicel showing expanded calyx and the verrucate, turbinate, fleshy pedicel; F flower, petal removed to show stamens and carpels on torus; G hairs on outer surface of petals; H calyx, showing verrucate surface, indumentum, and a minute vestigial sepal lobe; J fruit, reconstructed; K seed, side view; L seed, transverse section. A – H from Luke et al. 11205; J -– L from Luke et al. 9526. drawn by andrew brown.
Dagallier’s study shows Dennettia Baker f. (sunk into Uvariopsis by Schatz (Kenfack et al. 2003)), as sister to Monocyclanthus, supporting our resurrection of it as a distinct genus.
Twenty-seven genera are accepted in the Flora of Tropical East Africa account of Annonaceae, with 80 named species, and a further 11 which remained informally named (Verdcourt 1971). Lukea keys out in Verdcourt’s key to genera for flowering material, to couplet seven, since the carpels are free, and the hairs are simple. However, it fits neither arm of the couplet. While it has three valvate petals, it is not a tree, nor are the carpels 1-ovuled (Enantia Oliv., now Annickia Setten & Maas). Applying the key to fruiting material, it keys to couplet 54 because it has glabrous, non-tuberculate, several-seeded monocarps lacking longitudinal ribs with short stipes, seeds lacking pitted faces and far smaller than 2.5 cm long, leaves subglabrous. At this couplet it fails to fulfil both of the two statements since the fruiting pedicel (c. 1.5 mm long), neither exceeds 3 mm (leading to couplet 55) nor is the “fruit practically sessile” (the first arm: Hexalobus A.DC.). Further, in Hexalobus the petals are transversely plicate and united at the base, differing from those of Lukea which are unfolded and free.
In the fifty years since publication of the Flora of Tropical East Africa account (Verdcourt 1971), a further 30 additional new species have been described, bringing the cumulative total of formally named species of Annonaceae to 110 (Vollesen 1980; Verdcourt 1986; Verdcourt & Mwasumbi 1988; Johnson et al. 1999; Deroin & Luke 2005; Couvreur et al. 2006; Couvreur et al. 2009; Couvreur & Luke 2010; Hoekstra et al. 2016; Marshall et al. 2016; Johnson et al. 2017; Johnson & Murray 2018; Gosline et al. 2019; Dagallier et al. 2021; Hoekstra et al. 2021). It seems likely that additional species will continue to emerge for many years so long as taxonomists and botanical inventory work proceed and natural habitat survives and is incompletely surveyed.
Here we formally describe the new genus as Lukea Cheek & Gosline including two new species L. quentinii Cheek & Gosline (Kenya) and L. triciae Cheek & Gosline (Tanzania). We compare Lukea morphologically with the genera in the Uvariopsis clade: Uvariodendron, Uvariopsis, Mischogyne, Monocyclanthus and Dennettia.
Lukea increases to seven the number of endemic Annonaceae genera in the Eastern Arc Mts of Tanzania and the Kenyan Coastal Forests, the others being Asteranthe Engl. & Diels, Lettowianthus Diels, Mkilua Verdc., Mwasumbia Couvreur & D.M.Johnson, Ophrypetalum Diels, and Sanrafaelia Verdc.
Materials and Methods
Herbarium citations follow Index Herbariorum (Thiers, continuously updated). Specimens were viewed at EA (using http://eaherbarium.museums.or.ke/eaherbarium/results accessed 28 April 2021), BR, K, MO (https://www.tropicos.org/home accessed 28 April 2021). All specimens cited have been seen by one or more of the authors.
Binomial authorities follow the International Plant Names Index (IPNI continuously updated). The conservation assessment was made using the categories and criteria of IUCN (2012). Spirit preserved material was available of both species. Herbarium material was examined with a Leica Wild M8 dissecting binocular microscope fitted with an eyepiece graticule measuring in units of 0.025 mm at maximum magnification. The drawing was made with the same equipment using Leica 308700 camera lucida attachment. The flowers and fruits of herbarium specimens of the new species described were soaked in warm water to allow dissection. The terms and format of the description follow the conventions of Beentje & Cheek (2003) and Gosline et al. (2019). Georeferences for specimens lacking latitude and longitude were obtained using Google Earth (https://www.google.com/intl/en_uk/earth/versions/ ). The map was made using QGIS (https://www.qgis.org).
Taxonomic Results
Morphological characters separating Lukea from other genera in the Uvariopsis clade of Monodoreae are given in Table 1 below. Lukea, apart from the fleshy turbinate pedicel (a potential synapomorphy within the tribe which may also occur independently in the unrelated Neotropical genus Fusaea (Baill.) Saff. (Chatrou & He 1999)) has a combination of characters seen in no other genus.
Key to genera of the Uvariopsis clade
-
1. Quaternary nerves of leaf blades moderately or very conspicuous, forming a fine reticulum; petals & sepals 3-merous; flowers bisexual………………………………………………………………………...………………….…..2
-
1. Quaternary nerves of leaf blades absent or inconspicuous; petals (4) and sepals (2) 2-merous; flowers unisexual (except U. bisexualis)...……………………………………………………………………….....................……Uvariopsis
-
2. Inflorescences extra-axillary; bracts reduced to hair-tufts; calyx lobes covering corolla in bud; petals biseriate, strongly reflexed at anthesis……………………………………………………………………………….Mischogyne
-
2. Inflorescence axillary (or sometimes cauliflorous); bracts not reduced to hair tufts but fully developed, 3 – 4, distichous; calyx lobes if present, not covering corolla in bud; petals uniseriate, if reflexed, incompletely and slightly so………………………………………………………………………………………………...........………… 3
-
3. Stems non-flexuose; calyx caducous; carpels 7 – 80, calyx lobes moderately conspicuous ……..........……………4
-
3. Stems flexuose; calyx persistent in fruit; carpels 1 – 7, calyx lobes absent………………………………………….5
-
4. Corolla biseriate ………………………………………………………………………………...............Uvariodendron
-
4. Corolla uniseriate…………..………….…………………………………………………..…………………. Dennettia
-
5. Petals 6; pedicel cylindric; leaves with translucid gland dots, citrus scented (crushed); torus 2 – 3:1; carpels 6 – 7……………………………………………………………………………………………………..…..Monocyclanthus
-
5. Petals 3; pedicel turbinate; leaves without translucid gland dots, not citrus scented; torus 0.25:1; carpels 1 – 4………………………………………………………………………………………………………......………...Lukea
Lukea Cheek & Gosline gen. nov. Type species: Lukea triciae Cheek & Gosline
http://www.ipni.org/urn:lsid:ipni.org:names:77300187-1
Hermaphrodite evergreen shrubs. Leafy stems distichous, producing flushes of 2 – 4 leaves per season terminating in a dormant bud; bud-scales orbicular-ovate; stems terete, often flexuose (zig-zag), first (current) season’s growth drying green, lacking ridges and lenticels, indumentum sparse, hairs of 1 or 2 size-classes, simple, translucent, erect; axillary buds dome-shaped, of numerous filamentous, densely hairy scales. Second season’s epidermis greyish-black, with low, sinuous longitudinal ridges; lenticels inconspicuous, concolorous, raised, longitudinally elliptic, with a median longitudinal fissure; glabrescent. Leaves persisting for two seasons or more, stage-dependent heteromorphic (L. quentinii): those of sterile (non-reproductive) stems longer and larger than those of reproductive stems. Leaf-blades drying green, lacking translucid gland dots, subglossy or glossy above and matt below, ovate, elliptic or elliptic-oblong, rarely slightly oblanceolate, acumen short, broadly triangular, base rounded; midrib cylindric on the abaxial surface, visible usually only as a slit on the upper surface. Secondary nerves 6 – 19 on each side of the midrib, brochidodromous, arising at c. 60 – 80° from the midrib, straight, forking at c. 1 cm from the margin and uniting with the secondary nerves above and below to form an angular inframarginal nerve, connected by lower order nerves to two other parallel and less distinct, inframarginal nerves closer to the thickened, revolute margin. Tertiary and quaternary nerves also raised, as prominent as the secondary nerves, and forming a conspicuous reticulum of isodiametric cells on both adaxial and abaxial surfaces, cell (areolae) diam. c. 1 mm. Indumentum absent or sparse and inconspicuous, along the abaxial midrib and margin, hairs simple, appressed, white. Petioles slightly twisted, dark green (live) drying dull or bright orange, terete but the adaxial surface with a median slit, narrower in width at base and apex, transversely ribbed, glabrous.
Inflorescences, axillary, scattered sparsely along leafy or leafless nodes of stems 2 – 4 seasons old, 1-flowered, often (Lukea quentinii) with a second inflorescence arising sequentially from the base of the first. Peduncle and rachis together very short, hairs scattered, simple, dark brown, appressed. Bracts 3 – 4, distichous, green, persistent, shortly ovate-elliptic, apex acute or mucronate, base sheathing or not, increasing in size towards the pedicel, hairs sparsely scattered. Pedicel fleshy-leathery, yellow-green, turbinate, widening steadily from base to apex, the hairs as in the bracts. Flowers, bisexual. Calyx-receptacular tube, thick, leathery, externally warty, broad and short, shallow bowl-shaped, partly enclosing corolla in bud, indumentum on outer surface of scattered, dark brown, simple hairs, inner surface glabrous. Calyx lobes absent or vestigial, 3, alternating with the petals, triangular, minute, inserted at the edge of the calyx-receptacular tube aperture, centripetal (directed towards the floral axis), persistent (if developed), indumentum as receptacle but denser. Petals 3, green, valvate, thick and fleshy, shallowly concave, appearing nearly flat (horizontal) in bud (petal axis nearly perpendicular to that of floral axis), at anthesis elevated from the horizontal by 40 – 70°, triangular, apex acute, base truncate-retuse, sides square, outer surface densely covered in appressed sericeous golden hairs, inner surface glabrous. Torus (raised surface of receptacle on which are inserted the stamens and carpels), domed, wider than long. Stamens c. 150 – 180, spirally inserted, arranged in 8 – 12 ranks. Stamens latrorse, basifixed, erect, linear, connective and thecae nearly the length of the stamen, connective terminal extension semi-globose (fide van Heusden 1992), black, forming an incomplete cap over the anther cells. Pollen in tetrads. Petals and stamens falling after anthesis leaving the calyx-receptacle cup and carpels exposed, protected in the calyx-receptacular tube. Carpels (1 –) 2 – 4, brown, not diverging from each other, longer than stamens, subcylindrical, densely brown pubescent, the hairs patent. Stigma sessile, horseshoe shaped-crescentic, adaxial surface glabrous, outer surface with minute white patent hairs. Ovules numerous, biseriate, parietal. Fruit monocarps 1 (– 2) (numbers per fruit unknown in L. triciae), obovoid, subcylindric or narrow ellipsoid, in dried fruit becoming constricted or not between the seeds, pericarp yellow when mature, leathery, glossy, glabrous or very sparsely hairy; shortly stipitate or sessile, glabrous or glabrescent; apex rounded, obscurely bifurcate. Fruiting pedicels slightly accrescent. Calyx-receptacle and bracts persistent, green, not accrescent. Seeds 3 – 12 per monocarp, orange-brown discoid, or pale-yellow oblong-elliptic and widest near hilum, hilum large, elliptic, with a ragged, crater-like rim, raphe slightly raised, girdling the seed, testa bony, lacking pitting or sculpture; endosperm conspicuously lamelliform ruminate in longitudinal section, embryo flat, minute.
recognition. Similar to Monocyclanthus Keay in the flexuose stems, conspicuous fine quaternary reticulate venation, axillary, 1-flowered inflorescences (sometimes cauliflorous in Monocyclanthus), bisexual flowers, and calyx-receptacle with only rudimentary (or no) lobes, persistent and disc-like in fruit; differing in leaves lacking translucid gland dots and Citrus scent, torus broader than long (vs twice or more as long as broad), petals 3 (vs 6), the turbinate fleshy pedicel (vs cylindric), carpels 1 – 4 per flower (vs 6 – 7).
distribution. SE Kenya, Kilifi County and Tanzania, Morogoro Distr. The distribution of Lukea, with one species in the Eastern Arc Mts and another in the coastal forests of Kenya is seen in several other genera, such as Ancistrocladus Wall. with A. tanzaniensis Cheek & Frim.-Møll. in the Udzungwas and A. robertsoniorum J.Léonard in the Kenyan coastal forests (Cheek et al. 2000; Cheek 2000), also in the genus Afrothismia with A. mhoroana Cheek in the Ulugurus and A. baerae Cheek in Kenyan coastal forests (Cheek 2004a, Cheek 2006; Cheek & Jannerup 2006). Numerous other taxa are restricted to the Eastern Arc Mts of Tanzania and the Kenyan Coastal Forests, which together are referred to as EACF (see discussion).
habitat. Evergreen lowland forest remnants, c. 50 – 300 m elevation.
conservation status. Both species are globally threatened (see below under the species accounts).
etymology. Named for Mr and Mrs Luke, the Kenyan couple who together have collected many thousands of herbarium specimens in surveys they have organised of surviving natural habitat, principally in Kenya and Tanzania, including between them almost all of the material of this new genus.
notes. The monotypic Sanrafaelia Verdc. from the Usambara foothills of Tanzania shares a number of morphological similarities with Lukea: shrub habit, ribbed petioles, short pedicel with multiple overlapping bracts, connate sepals, and reduced anther connectives. Previously also included in Monodoreae, it is now placed in a separate tribe and can easily be distinguished in flower by having inner and outer petals both present and united in a single whorl (not with only the outer whorl present and free) (Dagallier 2021).
In our pre-print of this paper we had designated Lukea quentinii as type of the genus (Cheek et al. 2021), but as a precautionary measure change this here to L. triciae as only the last was included in the molecular study of Dagallier (2021). However, the two species are so closely similar morphologically that initially they were considered to be one species. The characters separating them are quantitative and not qualitative.
Local names and uses of the species of Lukea are unknown and may not exist since both species are so rare. The pollination biology is also unknown, but we conjecture that beetles may be involved as is usual in Annonaceae in Africa since the petals remain largely closed at anthesis, forming a pollination chamber around the sexual parts (Gottsberger et al. 2011; Gottsberger 2012). Whether thermogenesis occurs as in Uvariodendron (Gottsberger et al. 2011) remains to be discovered.
Placement of Lukea in the Uvariopsis clade
The Uvariopsis clade of the Monodoreae (consisting of the African genera Uvariodendron, Uvariopsis, Mischogyne, Dennettia and Monocyclanthus) is characterised (Table 1) by a tendency (present in most but not all genera of the clade) to conspicuous, finely reticulate quaternary nervation in the leaf-blades (usually absent or inconspicuous in Annonaceae) and incomplete or absent connective shields (in Annonaceae the connective shield is usually complete and is considered plesiomorphic). Within the Monodoreae, the Uvariopsis clade is further characterised by having free petals (not united as in the other clade of the tribe, the Isolona clade (Dagallier 2021)), however, a few species of Uvariopsis do have united petals. Placement of Lukea with this group is supported by possession of all three of these characters. The characters are not seen in all genera of the clade, e.g., in Uvariopsis the fine reticulation is not conspicuous, and a connective shield is present in both Monocyclanthus and Dennettia. The last genus has been sunk into Uvariopsis (Kenfack et al. 2003) but is maintained as a separate genus here since it differs in having a connective shield, and in combining two characters (trimerous perianth and bisexual flowers) otherwise seen separately only in two atypical species of Uvariopsis (Uvariopsis congolana (De Wild.) R.E.Fr., and U. bisexualis Verdc., respectively). Dagallier’s phylogenetic trees show Dennettia as sister to Monocyclanthus with high levels of support. Despite Lukea being placed in the Uvariopsis clade as sister to Mischogyne (Dagallier 2021), it lacks the numerous diagnostic features of that genus within the clade, such as the extra-axillary inflorescences, the calyx-sheathed corolla in bud, the strongly reflexed corolla with exserted carpels and the extremely long torus (length: breadth 3+:1) (Gosline et al. 2019). Similarities are the reticulate venation, the stamens without connective shield, and the ellipsoid monocarps in the fruit.
Morphologically the new genus appears closest to the West African monotypic Monocyclanthus, sharing a trait unusual in the Annonaceae: the calyx in both genera forms a shallow bowl (calyx lobes are absent or vestigial), barely enclosing even the base of the petals at anthesis. The bowl persists, green and leathery in the mature fruit (Fig. 2). A broadly similar structure is found in Uvariodendron schmidtii Q.Luke, Dagallier & Couvreur (Dagallier et al. 2021) in which the three calyx lobes are distinct, but appear almost completely connate (the individual lobes can still be clearly discerned) forming a shallow saucer at anthesis, however this structure falls post-anthesis (Dagallier et al. 2021) unlike in Lukea. The ancestor of Lukea may have had a similar calyx, which we speculate may have evolved by developing greater concavity, more complete loss of the calyx lobes, and persistence in the fruit. The occasional presence of vestigial inner petals in Lukea also suggest a link with Mischogyne and Uvariodendron which alone in the clade have regular inner petals. The function of the calyx-like structure, which may derive more from the enlarged receptacle than the calyx (suggested by the lack of evident calyx lobe connation) apart from protecting the flower in early bud, is unknown. It may be that the same structure in Monocyclanthus arose independently, and that the close similarity with Lukea does not indicate a sister relationship. Monocyclanthus and Lukea also share flexuose leafy twigs (seen intermittently in genera of other tribes e.g. Mwasumbia), and an unusually low number of carpels per flower (1 – 4 in Lukea and 6 – 7 in Monocyclanthus).
Key to the species of Lukea
-
Stems of leafy reproductive shoots thinly but softly white hairy, hairs patent, 0.1 – 0.2 mm long; leaves ovate-elliptic, (4.5 –) 6.8 – 10.5 × (2.5 –) 4 – 6 cm, lateral nerves 5 – 7 on each side of the midrib; petals 5 – 6 mm wide; fruit stipitate, cylindric, constricted between the seeds when dry. Coastal Kenya (Kaya Ribe)…...............................................................................................................................................................................................1. Lukea quentinii
-
Stems of leafy reproductive shoots glabrescent, hairs inconspicuous, 0.05 mm long; leaves ovate-elliptic, (11.2 –) 13.9 – 21 × (4.4 –) 5.2 – 7.4 (– 8.1) cm, lateral nerves 17 – 19 on each side of the midrib; petals 7 – 9 mm wide; fruit sessile, narrowly ellipsoid, not constricted between the seeds when dry. Tanzania (Morogoro Distr.)…...................................................................................................................................................................2. Lukea triciae
1. Lukea quentinii Cheek & Gosline sp. nov. Type: Kenya, Kilifi County, Kaya Ribe, Mleji River, 03°54'S, 39°38'E, fl., fr. 16 Jan. 1999, Q. Luke 5700 (holotype K000593320; isotypes EA000008943, MO, US).
http://www.ipni.org/urn:lsid:ipni.org:names:77300188-1
Hermaphrodite evergreen shrub c. 3 m tall. Leafy stems producing flushes of 3 – 4 leaves per season terminating in a dormant bud; bud-scales c. 4 – 7, orbicular 1.5 × 1.5 mm; stems terete, 2 – 3 mm diam. often flexuose (zig-zag), internodes 2 – 4.2 cm long, first (current) season’s growth drying green, lacking ridges and lenticels, indumentum moderately dense, covering 10 – 20% of the surface, hairs simple, white, erect, 0.1 – 0.2 mm long, intermixed with a few longer hairs, persisting for six internodes or more from the stem apex. Second season’s growth greyish-black, with low, sinuous longitudinal ridges; lenticels inconspicuous, concolorous, raised, longitudinally elliptic, 0.6 – 1.5 × 0.5 mm, with a median longitudinal fissure; glabrescent. Leaves persisting for two seasons or more, blades drying pale green, lacking glands, thickly papery, subglossy above and matt below, those of sterile (non-reproductive) stems (Luke 4703, K) longer than those of reproductive stems, elliptic or elliptic-oblong, rarely slightly oblanceolate, 11 – 15 × 5.5 – 7.8 cm, acumen short, broadly triangular, c. 0.4 × 0.4 cm, base rounded, minutely and abruptly cordate at the midrib; midrib cylindrical, 1 – 1.2 mm diam. on the abaxial surface, visible only as a slit on the upper surface, densely patent hairy, glabrescent. Secondary nerves 7 – 8 on each side of the midrib, brochidodromous, arising at c. 80° from the midrib, straight, forking at c. 1 cm from the margin and uniting with the secondary nerves above and below to form an angular inframarginal nerve, connected by lower order nerves to two other, less distinct, inframarginal nerves closer to the thickened, revolute margin. Tertiary and quaternary nerves also raised, as prominent as the secondary nerves, and forming a conspicuous reticulum on both adaxial and abaxial surfaces. Indumentum sparse and inconspicuous, along the abaxial midrib and abaxial blade towards the margin, hairs simple, appressed, white, 0.1 (– 0.3) mm long. Petioles (4.5 –) 6 mm long, (1.5 –) 2.5 mm wide. Leaves of reproductive stems (Luke 4740, 5700, K) similar to but smaller and differently shaped to those of the sterile stems, ovate or ovate-elliptic, (4.5 –) 6.8 – 10.5 × (2.5 –) 4 – 6 cm, lateral nerves 5 – 7 (– 8) on each side of the midrib. Petioles cylindric 3 × 1.3 mm, sparsely covered (5 – 10% of surface) with patent white hairs 0.3 mm long. Inflorescences, axillary, scattered sparsely along leafy or leafless nodes of stems 2 – 4 seasons old, 1-flowered, often with a second inflorescence arising sequentially from the base of the first. Peduncle and rhachis together 0.5 – 1 × 0.5 – 1 mm at anthesis, hairs dense becoming scattered with age, simple, dark brown or bronze, 0.2 – 0.5 mm long. Bracts 3 – 4, distichous, green, persistent, shortly ovate-elliptic, 1.2 – 1.5 × 1.2 – 1.5 mm apex acute or mucronate, base sheathing, increasing in size towards the pedicel, hairs scattered, red-brown 0.1 – 0.2 mm long. Pedicel turbinate, fleshy-leathery, yellow-green, widening steadily from base to apex, the base 1.5 mm diam., apex 3.5 mm diam., hairs as bracts. Flowers, bisexual. Calyx-receptacular tube mostly enclosing corolla in bud, bowl-shaped, 2 – 3 mm long, 6 – 8 mm wide, distal aperture orbicular, 6 – 8 mm diam. at anthesis, indumentum on outer surface scattered, dark brown, simple 0.25 – 0.3 mm long, inner surface glabrous. Calyx absent or vestigial, lobes 3, small triangular 0.1 – 0.2 × 0.5 mm, inserted at the edge of the receptacular tube distal aperture, centripetal, indumentum as receptacle. Petals 3, alternating with sepals, green, valvate, thick and fleshy, slightly concave, appearing nearly flat (horizontal) in bud (petal axis nearly perpendicular to that of floral axis), at anthesis elevated from the horizontal by 40 – 50°, triangular, 5 × 5 – 6 mm, apex acute, base (when detached) truncate-retuse, sides square, 1.5 mm thick along the midline, outer surface densely covered in appressed sericeous golden hairs. Vestigial inner petal sometimes present, oblanceolate to spatulate, 2.5 × 0.75 mm, apex rounded, glabrous. Torus (raised surface of receptacle on which are inserted the stamens and carpels), domed, wider than long, 0.5 – 0.75 × 2 mm. Stamens c. 150, spirally inserted, arranged in 8 – 10 ranks. Stamens latrorse, basifixed, erect, linear 1.4 – 1.5 × 0.25 mm, filament 0.1 mm long, connective and thecae nearly the length of the stamen, connective terminal extension globose, 0.1 mm long, black, forming an incomplete cap over the anther cells. Petals and stamens dropping after anthesis leaving the calyx-receptacle cup and carpels exposed. Carpels (1 –) 2 (– 3) brown, longer than stamens, subcylindrical, 2.5 × 0.5 mm, densely brown pubescent, the hairs patent, 0.05 – 0.1 mm long. Stigma yellowish-white, sessile, horseshoe shaped-crescentic, 0.8 × 0.6 mm, adaxial surface glabrous, outer surface with minute white patent hairs 0.05 mm long. Fruit monocarps 1 (– 2), live: obovoid or subcylindric, 3.1 – 4.8 × 2.2 – 2.9 cm, dry: subcylindric, 1.8 – 2.3 (– 3.5) × 1.1 – 1.3 (– 1.5) cm, constricted c. 1 mm between the (3 –) 4 – 6 seeds, yellow, pericarp leathery, glossy, glabrous apart from a few simple hairs 0.2 mm long; stipe 1 – 1.5 × 2 mm, glabrous; apex rounded, minutely bifurcate. Fruiting pedicels slightly accrescent, 1.5 mm long. Calyx-receptacle and bracts persistent in fruit, green, not accrescent. Seeds embedded in white, mealy, sweet pulp (Fig. 2), 3 – 6 per monocarp, orange-brown, discoid, 10 – 12 mm diam. × 4 – 6 mm thick, margin rounded, testa 0.25 mm thick; hilum elliptic 4 – 6 × 1 – 2 mm, rim crater-like, 0.5 mm high, verrucose; endosperm ruminate, embryo transversely elliptic in transverse section, 4.5 × 2.5 mm (Figs 1 & 2). See also Table 2.
recognition. Similar to Lukea triciae Cheek & Gosline, distinct in the leaves of reproductive stems smaller ((4.5 –) 6.8 – 10.5 × (2.5 –) 4 – 6 cm long), ovate or ovate-elliptic with lateral nerves 5 – 7 (– 8) on each side of the midrib (vs (11.2 –) 13.9 – 21 × (4.4 –) 5.2 – 7.4 (– 8.1) cm), oblong, lateral nerves 17 – 19 on each side) and in the smaller flowers (petals 5 – 6 mm wide vs 8 mm wide), carpels (1 –) 2 (– 3) (vs 4); fruits constricted between the seeds when dry (vs not constricted between the seeds).
distribution. SE Kenya (Map 1). Endemic to the Kaya Ribe forest, Kilifi County.
specimens examined. kenya. Kilifi County, Kaya Ribe, 0354S, 3938E, 15 July 1997, Q. Luke 4703 (EA000008944, K000593319); ibid., Mleji R. fl. buds, fr. 16 Sept. 1997 Q. Luke & P. Luke 4740 (EA, K000593318, MO n.v.); ibid., fl., fr. 16 Jan. 1999, Q. Luke 5700 (holotype K000593320; isotypes EA000008943, MO, US n.v.); ibid., Kaya Ribe, Mbisini R., fl., fr. 30 May 2021, Q. Luke, D. Marahaba, M. Makenzi & P. Lungo s.n. (EA).
habitat. Evergreen to semi-deciduous coastal Kaya forest along two streams, favouring the top of the up to 5 m high stream banks, on a grey, shale-like substrate. Associated species are Mitriostigma greenwayi, Encephalartos hildebrandtii, Asteranthe sp. nov., Mkilua fragrans, Uvariodendron kirkii, Xylopia holtzii, Gyrocarpus americanus, Rinorea spp., Barringtonia racemosa, Combretum schumannii, Terminalia sambesiaca, Garcinia spp., Cola spp., Sterculia spp., Rhodognaphalon schumannianum, Euphorbia wakefieldii, Ricinodendron heudelotii, Aristogeitonia monophylla, Afzelia quanzensis, Bauhinia mombassae, Caesalpinia insolita, Cynometra spp., Dialium spp., Hymenaea verrucosa, Julbernardia magnistipulata, Newtonia paucijuga, Parkia filicoidea, Cordyla africana, Erythrina sacleuxii, Millettia spp., Celtis spp., Antiaris toxicaria, Ficus spp., Milicia excelsa, Trichilia emetica, Lecaniodiscus fraxinifolius, Lannea welwitschii, Sorindeia madagascariensis, Diospyros spp., Manilkara spp., Synsepalum spp., Breonadia salicina, Premna spp., Borassus aethiopum, Pandanus rabaiensis (Luke unpublished data). The forest is on a hill c. 90 m high that rises from a plain at 40 – 50 m alt.
conservation status. Kaya Ribe, the sole known location for Lukea quentinii, is one of the Kenyan coastal Kaya forests. It is a traditional sacred forest of the Mjikenda and in particular for the WaRibe tribe. It was gazetted as a National Monument (NM) in 1992 under the National Museums & Monuments Act. This has afforded it some protection but it still suffers from tree cutting and encroachment. It has also been listed with other Kayas as a World Heritage Site under UNESCO. From CNES/Airbus imagery dated 29 June 2020 available through Google Earth (viewed 8 May 2021, see 3.900 S 39.633E), the forest looks in reasonable condition although there appear to be several gaps, possibly from the threats mentioned above. In particular, since 2011 imagery shows that forest along the western stream, the Mleji, which runs for c. 1 km through the forest, bisecting it, appears to have reduced in coverage. A field visit in 2021 discovered the species also along the eastern stream, the Mbisini. These two forested stream edges are the main, possible the only, habitat of Lukea quentinii, The forest is about 1 km diam., with an area of about 3.14 km2, equating to both the area of occupancy and extent of occurrence of the species. The forest is surrounded by agricultural fields. Numerous other plant species of the Kenyan coastal forests are already Red Listed, e.g. Cola pseudoclavata Cheek, C. octolobioides Brenan (Cheek & Lawrence 2019; Luke et al. 2018 respectively). We here provisionally assess Lukea quentinii as Critically Endangered, CR B1+2ab(iii) given the metrics and threats stated above.
phenology. Sterile (lacking flowers and fruit) in July, flower buds present in Sept., open flowers, ripe and immature fruit in Jan. and some also (possibly out of season) in late May.
etymology. Named for Quentin Luke, noted Kenyan botanist, Research Associate of the East African herbarium (EA), William Richard Quentin Luke, better known as Quentin Luke (1952 –). He is the leading specialist on the plants of the Kenyan coastal forests, and has discovered numerous new species of plants especially in Kenya and Tanzania, such as the incredible spectacular Tanzanian tree acanth Barleria mirabilis I.Darbysh. & Q.Luke (Darbyshire & Luke 2016). He has also brought to light species from across Africa e.g., in Democratic Republic of Congo: Keetia namoyae O.Lachenaud & Q.Luke (Lachenaud et al. 2017) and from Mali and Guinea the only endemic African Calophyllum, C. africanum Cheek & Q.Luke (Cheek & Luke 2016; Couch et al. 2019). He has also collected and described many other novel Annonaceae from Tanzania and Kenya (see introduction). Ten species are named for him, including the Tanzanian species Cola quentinii Cheek (Cheek & Dorr 2007) and Cola lukei Cheek (Cheek 2002). He is the lead or sole collector of all known fertile material of this new species. For further biographical and bibliographical information see (Polhill & Polhill 2015: 276 – 277).
vernacular name & uses. None are recorded.
notes. The first known collected specimen of Lukea quentinii (Q. Luke 4703, K) is sterile. It has larger and longer leaves than the other two, fertile collections, and so we deduce that these leaves may represent a juvenile, or at least, a non-reproductive stage.
This species is extremely rare and localised. It represents an addition to the Flora of the Kenyan coastal forests, not being listed in Ngumbau et al. (2020).
Fruits with constrictions between the seeds are a character state for genera and species in Annonaceae, however at least in the case of Lukea quentinii it is clear that this is an artefact of drying (compare Fig. 1Q with Fig. 2).
Recent field observations (30 May 2021) by QL noted that some flowers occur on the trunk (cauliflorous), as well as being leaf axillary, or on leafless stems as previously recorded. This condition is not unknown elsewhere in the family, e.g. in Goniothalamus grandifloras Boerl. (Utteridge 2021).
2. Lukea triciae Cheek & Gosline sp. nov. Type: Tanzania, Morogoro Distr., Udzungwa Mts National Park, Msolwa - Camp 244, 7°43'S, 36°55'E, 350 m alt., fl. 23 Oct. 2005, W. R. Q. Luke, Mwangoka & Festo 11205 (holotype K000593317; isotypes EA000008947, MO, NHT).
http://www.ipni.org/urn:lsid:ipni.org:names:77300189-1
Hermaphrodite evergreen shrub or small tree 2 – 3 m tall. Leafy stems producing flushes of 2 – 3 leaves per season, terminating in a dormant bud; bud-scales 6 – 8, ovate 1.5 × 1.5 mm; stems terete, 2 – 3 mm diam. often flexuose (zig-zag), internodes 1.7 – 4 cm long, first (current) season’s growth drying green, lacking ridges and lenticels, indumentum glabrescent, inconspicuous, very sparse, simple, translucent, erect, >0.05 mm long. Second season’s growth greyish-black, with low, sinuous longitudinal ridges; lenticels inconspicuous, concolorous, raised, longitudinally elliptic, glabrescent. Leaves persisting for two seasons or more, blades drying coriaceous, elliptic-oblong, (11.2 –) 13.9 – 21 × (4.4 –) 5.2 – 7.4 ( – 8.1) cm, acumen c. 0.8 – 1.4 cm, base rounded; midrib cylindrical, 1 – 1.2 mm diam. on the abaxial surface, visible only as a slit on the upper surface. Secondary nerves 17 – 19 on each side of the midrib, brochidodromous, arising at c. 70 – 80° from the midrib, arching upwards, forking at 1 – 2 cm from the margin and uniting with the secondary nerves above and below to form an angular inframarginal nerve, connected by lower order nerves to two other, less distinct, inframarginal nerves closer to the thickened, revolute margin. Tertiary and quaternary nerves also raised, as prominent as the secondary nerves, and forming a conspicuous reticulum on both adaxial and abaxial surfaces. Indumentum sparse and inconspicuous, glabrescent. Petioles 5 – 9 mm long, (1.5 –) 2.5 mm wide. Inflorescences, axillary, scattered sparsely along leafy or leafless nodes of stems 2 seasons old, 1-flowered. Peduncle and rhachis together c. 1 mm long at anthesis, hairs sparse, simple. Bracts 3 – 4, distichous, persistent, shortly ovate, c. 1.5 × c. 1.5 mm apex rounded, base not-sheathing, increasing in size towards the pedicel, hairs scattered, red-brown 0.1 – 0.2 mm long. Pedicel turbinate, fleshy-leathery, yellow-green, widening steadily from base to apex, the 1.5 – 3.5 mm long, base 1.5 mm diam., apex 5 – 6 mm diam., glossy, subglabrous. Flowers, bisexual, known only from slightly pre-anthetic material. Calyx-receptacular tube mostly enclosing corolla in bud, bowl-shaped, 2 mm long, 8 mm wide, distal aperture orbicular, 6 – 8 mm diam. at anthesis, indumentum on outer surface scattered, dark brown, simple 0.25 – 0.3 mm long, inner surface glabrous. Calyx vestigial, lobes 3, triangular, 0.5 × 1 mm, hairs moniliform, simple, 0.25 – 0.3 mm long. Petals 3, valvate, thick and fleshy, slightly concave, appearing nearly flat (horizontal) in bud (petal axis nearly perpendicular to that of floral axis), not seen at anthesis, triangular, 5 – 6 × 8 mm, apex acute, base truncate-retuse, sides square, 1.5 mm thick, along the midline, outer surface densely covered in appressed sericeous golden hairs. Torus (raised surface of receptacle on which are inserted the stamens and carpels), not seen (material scarce). Stamens c. 150, spirally inserted, arranged in c. 12 ranks. Stamens latrorse, basifixed, erect, linear 1 × 0.25 mm, filament minute, connective and thecae nearly the length of the stamen, connective terminal extension globose, 0.2 × 0.05 mm, black, forming an incomplete cap over the anther cells. Carpels 4, longer than stamens, subcylindrical, not dissected due to shortage of material. Stigma sessile, horseshoe shaped-crescentic, 0.8 × 0.6 mm, adaxial surface glabrous, outer surface with minute white patent hairs 0.05 mm long. Fruit monocarps (number per fruit unknown), drying brown, pericarp leathery, glossy, ellipsoid, 3.8 × 2.1 cm, not constricted between the seeds, apex rounded, base sessile, glabrous. Peduncle-pedicel, calyx-receptacle and bracts not recorded in fruit. Seeds c. 12 per monocarp, pale-yellow, oblong, compressed, widest at hilum end, 11 – 12 × 6 – 8 × 3 – 4 mm, marginal raphe ridge apex acute, hilum sunken, elliptic, 4 × 3 mm, margin raised, with short fibres, central sunken area with longitudinal ridge 2 mm long, micropylar aperture conspicuous; testa 0.2 mm thick, bony; endosperm ruminate in longitudinal section, embryo flat, c. 3 mm wide. Figs 3 & 4.
recognition. Lukea triciae is similar to L. quentinii Cheek & Gosline, distinct in the leaves of reproductive stems being larger ((11.2 –) 13.9 – 21 × (4.4 –) 5.2 – 7.4 (– 8.1) cm), oblong, lateral nerves 17 – 19 on each side, (vs smaller ((4.5 –) 6.8 – 10.5 × (2.5 –) 4 – 6 cm long), ovate or ovate-elliptic with lateral nerves 5 – 7 (– 8) on each side of the midrib) and the larger flowers (petals 8 mm wide vs 5 – 6 mm wide), carpels 4 (vs (1 –) 2 (– 3)); fruits cylindric-ellipsoid, when dry not constricted between the seeds (vs constricted between the seeds). See also Table 2.
distribution. Tanzania (Map 1). Endemic to surviving lowland forest patches around the Udzungwa Mts
specimens examined. tanzania. Morogoro District. Mtibwa Forest Reserve, St. (Dec. 1953), Semsei 1520 (EA000008946, K000593315); Udzungwa Mts National Park, Msolwa (village)- Camp 244, 7°43'S, 36°55'E, 350 m alt., fl. 23 Oct. 2005, W. R. Q. Luke, Mwangoka & Festo 11205 (holotype K000593317; isotypes EA000008947, MO, NHT); Magombera Forest, Rogers & Homewood s.n., (EA); ibid., 07°48'S, 36°58'E, 250 m alt., fl. fr. 19 July 2003, Q. Luke, P. Luke & Arafat 9526 (EA, K000593316, MO3055171, NHT).
habitat. Evergreen lowland forest remnants; 250 – 350 m alt. At the Udzungwa Mts Msolwa location, Lukea triciae occurs on the bank of a small stream with riverine forest that runs through woodland at base of an escarpment. Associated species are Uvaria tanzaniae, Pteleopsis myrtifolia, Terminalia sambesiaca, Sterculia quinqueloba, Pseudolachnostylis maprouneifolia, Erythrophleum suaveolens, Isoberlinia scheffleri, Parkia filicoidea, Tetrapleura tetraptera, Dalbergia spp., Celtis zenkeri, Ficus spp., Milicia excelsa, Treculia africana, Blighia unijugata, Lecaniodiscus fraxinifolius, Sorindeia madagascariensis, Pouteria alnifolia, Strychnos spp., Schrebera trichoclada, Diplorhynchus condylocarpon, Holarrhena pubescens, Catunaregam spp., Crossopteryx febrifuga, Borassus aethiopum.
At the Magombera Forest Reserve site, it was found in lowland ?groundwater forest with Huberantha verdcourtii, Isolona heinsenii, Uvaria tanzaniae, Rinorea spp., Memecylon magnifoliatum, Cola spp., Rhodognaphalon schumannianum, Drypetes parvifolia, Erythrophleum suaveolens, Guibourtia schliebenii, Hymenaea verrucosa, Julbernardia magnistipulata, Albizia gummifera, Parkia filicoidea, Dalbergia spp., Pterocarpus mildbraedii, Ficus spp., Treculia africana, Pancovia holtzii, Sorindeia madagascariensis, Diospyros spp., Noronhia mildbraedii, Aoranthe penduliflora, Coffea spp., Craterispermum schweinfurthii, Psychotria spp., Vitex doniana, Borassus aethiopum.
conservation status. The site of the earliest known collection of this genus and of the species Lukea triciae, is Mtibwa Forest Reserve (located at 6°07'S, 37°39'E according to Polhill 1988). According to https://www.tfs.go.tz/index.php/en/forests/mtibwa (accessed 21 April 2021), it is now a forestry plantation, 95% being teak, Tectona grandis L.f., and only a few small scraps of indigenous vegetation remain on land unsuitable for plantation. This can be confirmed by viewing through Google Earth using the georeference provided above where the former forest is now divided into parcels of planted trees. It seems likely, even certain, that the species has become extinct at this location where it was collected by Semsei in 1953, however a targeted search at Mtibwa is advised to confirm local extinction before this is accepted.
Lukea triciae is therefore now on current evidence confined to possibly three (but probably only two) locations separated from each other by 12 km of cultivated land at the foot of the Udzungwa Mts. Since there are only two collections with good geographical metadata, one from each site, we estimate the maximum area of occupation as being 12 km2 using the grid cells of 4 km2 preferred by IUCN (2012) and the extent of occurrence as 868 km2.
The Magombera Forest Reserve site where the species was discovered in 2003, was highly threatened when it was degazetted from the Selous Game Reserve (now also known as the Nyerere National Park) and not regazetted immediately as was intended at the time. It was part privately owned by the adjacent sugar estate that cleared much of the previous forest in the adjoining Kilombero valley in the 1970s. (https://www.rainforesttrust.org/urgent-projects/creating-the-magombera-nature-reserve-in-tanzania/ Accessed 8 August 2022). The Tanzania-Zambia railway was also cut through the forest in the 1970s. A recent campaign to have Magombera given special protection has been successful. It was gazetted as a Nature Reserve in 2019 and is now subject to fundraising. Since 2008 (imagery viewed via Google Earth) some patches of forest at the margin have been cleared, presenting a threat to the habitat of Lukea triciae. Threats at the Msolwa site are firewood collection (Luke pers. obs.). Assuming that the Mtibwa location has been lost, EOO, AOO and mature individuals have therefore also been lost. Given this, the threats above and small EOO and AOO, we here assess the species as EN B1ab(i-v), B2ab(i-v).
phenology. Flowers and fruit in July, flower buds in Oct., sterile in December.
etymology. Named for key co-collector of this new species, Patricia (better known as Tricia) Luke. She organised the botanical fieldwork logistics for the Udzungwa mountains survey during which flowering and fruiting herbarium material came to light in 2003 and 2005. These specimens were crucial for providing the samples that enabled the molecular phylogenetic placement of the new genus. She has organised the logistics for numerous other botanical surveys and has had a life-long interest in nature and conservation, especially in her native Kenya.
vernacular name & uses. None are recorded.
notes. Lukea triciae is more incompletely known than L. quentinii. Only a single monocarp is known, and the few flowers available are not quite at anthesis. The earliest gathering, (Semsei 1520, K, sterile) has leaves of similar size and shape to those of fertile collections and so there is no evidence that there is a different juvenile or non-flowering stage, as seen in L. quentinii (see above). Lukea triciae is confined to Morogoro District, Tanzania, to which numerous other rare and threatened species are globally endemic, e.g. Brachystephanus schleibenii (Mildbr.) Champl. (Champluvier & Darbyshire 2009), and Keetia semsei Cheek & Bridson (Cheek & Bridson 2019) both known from a single collection, and Asystasia asystasioides Darbysh. & Ensermu (Darbyshire & Ensermu 2007).
Discussion
The Eastern Arc Mountains & Coastal Forests
The two species are separated geographically by c. 525 km, as measured by Google Earth. Both locations are placed in the Eastern Arc mountains and Coastal Forests (EACF). The EACF are found in Tanzania and southern Kenya. They form an archipelago-like phytogeographical unit well-known for high levels of species endemism in many groups of organisms (Gereau et al. 2016). Among the better-known mountain blocks are the Udzungwas, the Ulugurus, and the Usambaras. The Mtibwa Forest reserve at the foot of the Nguu Mts, home of Lukea triciae, is among the least known, least well surveyed, and least protected forest patches. Supported by moist air currents from the Indian Ocean, the surviving evergreen forests of the Eastern Arc Mountains alone have 223 species of endemic tree (Lovett 1998) and are variously stated to have 800 (Tanzanian Forest Conservation Group, undated) or as many as 1500 species (Skarbek 2008) of endemic plant species. In herbaceous groups such as the Gesneriaceae, over 50% of the species for East Africa (Uganda, Kenya and Tanzania) are endemic to the Eastern Arc Mts (Darbyshire 2006) and in the Acanthaceae, there are as many as 100 species endemic to the Eastern Arc Mts (Darbyshire et al. 2010) e.g. Isoglossa bondwaensis I.Darbysh. endemic to the Uluguru Mts (Darbyshire 2009). In terms of documented plant species diversity per degree square, the Eastern Arc Mts are second only in tropical Africa to SW Cameroon in the Cross-Sanaga Interval of W-C Africa (Barthlott et al. 1996; Cheek et al. 2001), which also has the degree square with highest generic diversity (Dagallier et al. 2020). Lukea is an example of a genus endemic to the EACF, just as e.g. Medusandra Brenan (Peridiscaceae, Soltis et al. 2007; Breteler et al. 2015) is endemic to the Cross-Sanaga Interval. Several forest genera have disjunct distributions, being found only in the Cross-Sanaga Interval and in the EACF and not in between, e.g., Zenkerella Taub. and Kupea Cheek (Cheek et al. 2003; Cheek 2004b). The EACF include the sole representatives of plant groups otherwise restricted on the continent to the forests of Guinea-Congolian Africa, e.g., Afrothismia Schltr. and Ancistrocladus Wall. (Cheek & Jannerup 2006; Cheek et al. 2000). Extensive forest clearance within the last 100 – 150 years has removed forest from some mountains entirely, and reduced forest extent greatly in others. Since the 1970s more than 12% of these forests have been cleared (Tanzania Forest Conservation Group, undated). Yet new taxa such as these two Lukea species are still being published steadily. Let us hope that their natural sites where not already destroyed can be preserved and their extinction avoided.
Conclusions
Lukea quentinii and L. triciae underline the urgency for identifying and publishing species while they still survive. Threats to such new discoveries for science are ever present, putting these species at high risk of extinction. About 2000 new species of flowering plant have been published by science each year for the last decade or more (Cheek et al. 2020), adding to the estimated 369,000 already documented (Nic Lughadha et al. 2016), although the number of flowering plant species known to science is uncertain (Nic Lughadha et al. 2017). Only 7.2% of species have been assessed and included on the Red List using the IUCN (2012) standard (Bachman et al. 2019), but this number rises to 21 – 26% when additional evidence-based assessments are considered, and 30 – 44% of these assess the species as threatened (Bachman et al. 2018). Newly discovered species, such as those reported in this paper, are likely to be threatened, since widespread species tend to have been already discovered. There are notable exceptions to this rule (e.g. Vepris occidentalis Cheek (Cheek et al. 2019) a species widespread in West Africa from Guinea to Ghana). However, it is generally the more localised, rarer species that remain unknown to science. This makes it all the more urgent to find, document and protect such species before they become extinct. Until species are delimited, described and known to science, it is difficult to assess them for their IUCN conservation status and so the possibility of protecting them is reduced (Cheek et al. 2020). Documented extinctions of plant species are increasing, e.g., in coastal forest of Kenya Cynometra longipedicellata Harms may well now be extinct at its sole locality, the Amani-Sigi Nature Reserve (Gereau et al. 2020) and in Tanzania Kihansia lovettii Cheek (Cheek 2004b) at the Kihansi dam site, has not been seen since it was first collected despite targeted searches. There are also examples of species that appear to have become extinct even before they are known to science, such as Pseudohydrosme bogneri in Gabon (Moxon-Holt & Cheek 2021) and in Cameroon Vepris bali Cheek (Cheek et al. 2018). In all cases anthropogenic pressures have been the cause of these extinctions. In both Kenya and Tanzania, on the whole, natural habitat is relatively well covered by a well-planned network of protected areas, but nevertheless natural habitat at some sites with species of high value for conservation has all but disappeared as for the site for first collection of Lukea triciae at Mtwiba (see above). Further investment in prioritising the highest priority areas for plant conservation as Tropical Important Plant Areas (TIPAs, using the revised IPA criteria set out in Darbyshire et al. (2017)) as is in progress in countries such as Guinea, Cameroon, Mozambique and Ethiopia might be extended elsewhere to reduce further the risk of future global extinctions of range-restricted endemic species such as Lukea quentinii and L. triciae.
Change history
29 September 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12225-022-10056-x
References
Bachman, S. P., Nic Lughadha, E. M. & Rivers, M. C. (2018). Quantifying progress towards a conservation assessment for all plants. Conservation Biol. 32 (3): 516 – 524. https://doi.org/10.1111/cobi.13071
____, Field, R., Reader, T., Raimondo, D., Donaldson, J., Schatz, G. E. & Nic Lughadha, E. M. (2019). Progress, challenges and opportunities for Red Listing. Biol. Conservation 234: 45 – 55. https://doi.org/10.1016/j.biocon.2019.03.002
Beentje, H. & Cheek, M. (2003). Glossary. In: H. Beentje (ed.), Flora of Tropical East Africa. Balkema, Lisse. https://doi.org/10.1201/9781482283808
Barthlott, W., Lauer, W. & Placke, A. (1996). Global distribution of species diversity in vascular plants: Towards a world map of phytodiversity (globale verteilung der artenvielfalt höherer pflanzen: Vorarbeiten zu einer weltkarte der phytodiversität). Erdkunde 50: 317 – 327 (with supplement and figure). https://doi.org/10.3112/erdkunde.1996.04.03
Breteler, F. J., Bakker, F. T. & Jongkind, C. C. (2015). A synopsis of Soyauxia (Peridiscaceae, formerly Medusandraceae) with a new species from Liberia. Pl. Ecol. Evol. 148: 409 – 419. https://doi.org/10.5091/plecevo.2015.1040
Champluvier, D. & Darbyshire, I. (2009). A revision of the genera Brachystephanus and Oreacanthus (Acanthaceae) in tropical Africa. Syst. Geogr. Pl. 79: 115 – 192
Chatrou, L. W. & He, P. (1999). Studies in Annonaceae XXXIII. A revision of Fusaea (Baill.) Saff. Brittonia 51 (2): 181 – 203.
____, Erkens, R. H. J., Richardson, J. E., Saunders, R. M. K. & Fay, M. F. (2012a). The natural history of Annonaceae. Bot. J. Linn. Soc. 169: 1 – 4.
____, Pirie, M. D., Erkens, R. H. J., Couvreur, T. L. P., Neubig, K. M., Abbott, J. R., Mols, J. B., Maas, J. W., Saunders, R. M. K. & Chase, M. W. (2012b). A new subfamilial and tribal classification of the pantropical flowering plant family Annonaceae informed by molecular phylogenetics. Bot. J. Linn. Soc. 169: 5 – 40.
Cheek, M. (2000). A Synoptic Revision of Ancistrocladus (Ancistrocladaceae) in Africa, with a New Species from Western Cameroon. Kew Bull. 55: 871 – 882. https://doi.org/10.2307/4113632
____ (2002). A new species of Cola (Sterculiaceae) from the Usambara Mts, Tanzania. Kew Bull. 57: 417 – 422. https://doi.org/10.2307/4111119
____(2004a). A new species of Afrothismia (Burmanniaceae) from Kenya. Kew Bull. 58: 951 – 955. https://doi.org/10.2307/4111208
____ (2004b). Kupeaeae, a new tribe of Triuridaceae from Africa. Kew Bull. 58: 939 – 949. https://doi.org/10.2307/4111207
____ (2006). African Saprophytes: new discoveries. pp. 693 – 697. In: S. A. Ghazanfar & H. J. Beentje (eds), Taxonomy and Ecology of African Plants, their Conservation and Sustainable Use. Proceedings of the 17th AETFAT Congress, Addis Ababa, Ethiopia. Royal Botanic Gardens, Kew.
____ & Bridson, D. M. (2019). Three new threatened Keetia species (Rubiaceae—Vanguerieae), from the forests of the Eastern Arc Mountains, Tanzania. Gard. Bull. Singapore 71 (Suppl. 2): 155 – 169. https://doi.org/10.26492/gbs71(suppl.2).2019-12
____ & Dorr, L. (2007). Sterculiaceae. In: H. Beentje (ed.), Flora of Tropical East Africa. Royal Botanic Gardens, Kew.
____ & Jannerup, P. (2006). A new species of Afrothismia (Burmanniaceae) from Tanzania. Kew Bull. 60: 593 – 596. http://www.jstor.org/stable/25070246
____ & Lawrence, P. (2019). Cola pseudoclavata. The IUCN Red List of Threatened Species 2019: e.T111397669A111449482. https://doi.org/10.2305/IUCN.UK.2019-1.RLTS.T111397669A111449482.en [Downloaded 27 May 2019].
____ & Luke, Q. (2016). Calophyllum (Clusiaceae – Guttiferae) in Africa. Kew Bull. 71, 20. https://doi.org/10.1007/s12225-016-9637-6
____, Frimodt-Møller, C. & Hørlyck, V. (2000). A new submontane species of Ancistrocladus from Tanzania. Kew Bull. 55: 207 – 212. https://doi.org/10.2307/4117778
____, Gosline, G. & Onana, J-M. (2018). Vepris bali (Rutaceae), a new critically endangered (possibly extinct) cloud forest tree species from Bali Ngemba, Cameroon. Willdenowia 48: 285 – 292. https://doi.org/10.3372/wi.48.48207
____, Luke, W. R. Q. & Gosline, G. (2021). Lukea gen. nov. (Monodoreae-Annonaceae) with two new species of shrub from the forests of the Udzungwas, Tanzania & Kaya Ribe, Kenya. bioRxiv (pre-print). https://doi.org/10.1101/2021.05.14.444227
____, Mackinder, B. Gosline, G., Onana, J-M. & Achoundong, G. (2001). The phytogeography and flora of western Cameroon and the Cross River-Sanaga River interval. Syst. Geogr. Pl. 71: 1097 – 1100. https://doi.org/10.2307/3668742
____, Nic Lughadha, E., Kirk, P., Lindon, H., Carretero, J., Looney, B., Douglas, B., Haelewaters, D., Gaya, E., Llewellyn, T., Ainsworth, M., Gafforov, Y., Hyde, K., Crous, P., Hughes, M., Walker, B. E., Forzza, R. C., Wong, K. M. & Niskanen, T. (2020). New scientific discoveries: plants and fungi. Plants, People Planet 2: 371 – 388. https://doi.org/10.1002/ppp3.10148
____, Onana, J-M., Yasuda, S., Lawrence, P., Ameka, G. & Buinovskaja, G. (2019). Addressing the Vepris verdoorniana complex (Rutaceae) in West Africa, with two new species. Kew Bull. 74: 53. https://doi.org/10.1007/S12225-019-9837-Y
____, Williams, S. A. & Etuge, M. (2003). Kupea martinetugei, a new genus and species of Triuridaceae from western Cameroon. Kew Bull. 58: 225 – 228. https://doi.org/10.2307/4119366
Couch, C., Cheek, M., Haba, P. M., Molmou, D., Williams, J., Magassouba, S., Doumbouya, S. & Diallo, Y. M. (2019). Threatened habitats and Important Plant Areas (TIPAs) of Guinea, west Africa. Royal Botanic Gardens, Kew.
Couvreur, T. L. P. & Luke, W. R. Q. (2010). A new species of Uvariopsis (Annonaceae), endemic to the Eastern Arc Mountains of Tanzania. Blumea 55: 68 – 72. https://doi.org/10.3767/000651910X499196
____, Gereau, R. E., Wieringa, J. J. & Richardson, J. E. (2006). Description of four new species of Monodora and Isolona (Annonaceae) from Tanzania and an overview of Tanzanian Annonaceae diversity. Adansonia 28: 243 – 266.
____, Helmstetter, A. J., Koenen, E. J. M., Bethune, K., Brandão, R. D., Little, S. A., Sauquet, H. & Erkens, R. H. J. (2019). Phylogenomics of the Major Tropical Plant Family Annonaceae Using Targeted Enrichment of Nuclear Genes. Front. Pl. Sci. 9. https://doi.org/10.3389/fpls.2018.01941
____, van der Ham, R. W. J. M., Mbele, Y. M., Mbago, F. M. & Johnson, D. M. (2009). Molecular and morphological characterization of a new monotypic genus of Annonaceae, Mwasumbia, from Tanzania. Syst. Bot. 34 (2): 266 – 276. https://doi.org/10.1600/036364409788606398
Dagallier, L. P. (2021). Diversification of the tropical African flora: spatial and temporal approaches. Vegetal Biology. PhD thesis. Université Montpellier. English. ffNNT : 2021MONTG068ff. fftel-03599457f https://tel.archives-ouvertes.fr/tel-03599457
____, Janssens, S. B., Dauby, G., Blach-Overgaard, A., Mackinder, B. A., Droissart, V., Svenning, J. C., Sosef, M. S., Stévart, T., Harris, D. J., Sonké, B., Wieringa, J. J., Hardy, O. J. & Couvreur, T. L. P. (2020). Cradles and museums of generic plant diversity across tropical Africa. New Phytol. 225: 2196 – 2213. https://doi.org/10.1111/nph.16293
____, Mbago, F. M., Luke, W. R. Q. & Couvreur, T. L. P. (2021). Three new species of Uvariodendron (Annonaceae) from coastal East Africa in Kenya and Tanzania. PhytoKeys 174: 107 – 126. https://doi.org/10.3897/phytokeys.174.61630
Darbyshire, I. (2006). Gesneriaceae. Flora of Tropical East Africa. Royal Botanic Gardens, Kew.
____ (2009). Taxonomic notes and novelties in the genus Isoglossa (Acanthaceae) from east Africa. Kew Bull. 64: 401 – 427. https://doi.org/10.1007/s12225-009-9123-5
____ & Ensermu Kelbessa (2007). Isoglossa asystasioides, a striking new species of Acanthaceae from Tanzania. Kew Bull. 62: 617 – 621. https://www.jstor.org/stable/20443394
____, Anderson, S., Asatryan, A., Byfield, A., Cheek, M., Clubbe, C., Ghrabi, Z., Harris, T., Heatubun, C. D., Kalema, J., Magassouba, S., McCarthy, B., Milliken, W., Montmollin, B. de, Nic Lughadha, E., Onana, J-M., Saïdou, D., Sârbu, A., Shrestha, K. & Radford, E. A. (2017). Important Plant Areas: revised selection criteria for a global approach to plant conservation. Biodivers. Conserv. 26: 1767 – 1800. https://doi.org/10.1007/s10531-017-1336-6
____ & Luke, Q. (2016). Barleria mirabilis (Acanthaceae): a remarkable new tree species from west Tanzania. Kew Bull. 71, 13. https://doi.org/10.1007/s12225-016-9622-0
____, Vollesen, K. & Kelbessa, E. (2010). Acanthaceae (Part 2). Flora of Tropical East Africa. Royal Botanic Gardens, Kew.
Deroin, T. & Luke, W. R. Q. (2005). A new Toussaintia (Annonaceae) from Tanzania. J. E. Africa Nat. Hist. Soc. Natl. Mus. 94: 165 – 174. https://doi.org/10.2982/0012-8317(2005)94[165:ANTAFT]2.0.CO;2
Gereau, R. E., Cumberlidge, N., Hemp, C., Hochkirch, A., Jones, T., Kariuki, M., Lange, C. N., Loader, S. P., Malonza, P. K., Menegon, M., Ndang’ang’a, P. K., Rovero, F. & Shirk, P. (2016). Globally threatened biodiversity of the Eastern Arc Mountains and coastal forests of Kenya and Tanzania. J. E. African Nat. Hist. 105: 115 – 201. https://doi.org/10.2982/028.105.0104
____, Kabuye, C., Kalema, J., Kamau, P., Kindeketa, W., Luke, W. R. Q., Lyaruu, H. V. M., Malombe, I., Mboya, E. I., Mollel, N., Njau, E.-F., Schatz, G. E., Sitoni, D., Ssegawa, P. & Wabuyele, E. (2020). Cynometra longipedicellata. The IUCN Red List of Threatened Species 2020: e.T32275A2812477. https://doi.org/10.2305/IUCN.UK.2020-3.RLTS.T32275A2812477.en [Downloaded 1 May 2021].
Gosline, G., Marshall, A. R. & Larridon, I. (2019). Revision and new species of the African genus Mischogyne (Annonaceae). Kew Bull. 74: 28. https://doi.org/10.1007/s12225-019-9804-7
____, Cheek, M., Onana, J-M., Ngansop, E., van der Burgt, X. & Dagallier, L-P. (2021). Uvariopsis ebo (Annonaceae) a new, Critically Endangered tree species from the Ebo Forest, Cameroon and a key to the Cameroonian species of Uvariopsis. BioRxiv (pre-print). https://doi.org/10.1101/2021.03.26.437154
Gottsberger, G. (2012). How diverse are Annonaceae with regard to pollination? Bot. J. Linn. Soc. 169: 245 – 261. https://doi.org/10.1111/j.1095-8339.2011.01209.x
____, Meinke, S. & Porembski, S. (2011). First records of flower biology and pollination in African Annonaceae: Isolona, Piptostigma, Uvariodendron, Monodora and Uvariopsis. Flora — Morphology, Distribution, Functional Ecology of Plants 206: 498 – 510. https://doi.org/10.1016/j.flora.2010.08.005
Guo, X., Tang, C. C., Thomas, D. C., Couvreur, T. L. P. & Saunders, R. M. K. (2017). A mega-phylogeny of the Annonaceae: taxonomic placement of five enigmatic genera and support for a new tribe, Phoenicantheae. Sci. Rep. 7: e7323. https://doi.org/10.1038/s41598-017-07252-2
Heywood, V. H., Brummitt, R. K., Culham, A. & Seburg, O. (eds) (2007). Flowering Plant Families of the World. Royal Botanic Gardens, Kew.
Hoekstra, P. H., Wieringa, J. J. & Chatrou, L. W. (2016). A nonet of novel species of Monanthotaxis (Annonaceae) from around Africa. PhytoKeys 69: 71 –103. https://doi.org/10.3897/phytokeys.69.9292
____, ____, Maas, P. J. M. & Chatrou, L. W. (2021). Revision of the African species of Monanthotaxis (Annonaceae). Blumea 66 (2): 107 – 221. https://doi.org/10.3767/blumea.2021.66.02.01
The International Plant Names Index (continuously updated). http://ipni.org/. [Accessed May 2021].
IUCN (2012). IUCN Red List Categories and Criteria: version 3.1. Second edition. Gland and Cambridge. http://www.iucnredlist.org/ [Accessed May 2021].
Johnson, D. M. & Murray, N. A. (2018). A revision of Xylopia L.(Annonaceae): the species of Tropical Africa. PhytoKeys 97, 1.
____, Mwasumbi, L. B. & Mbago, F. M. (1999). New species of Xylopia and Uvaria (Annonaceae) from Tanzania. Novon 9: 55 – 60. https://doi.org/10.2307/3392119
____, Luke, Q., Goyder, D. J. & Murray, N. A. (2017). New species of Xylopia (Annonaceae) from East Africa. Kew Bull. 72, 11. https://doi.org/10.1007/s12225-017-9681-x
Kenfack, D., Gosline, G., Gereau, R. E. & Schatz, G. E. (2003). The genus Uvariopsis in Tropical Africa, with a recombination and one new species from Cameroon. Novon 13: 443 – 449. https://doi.org/10.2307/3393377
Lachenaud, O., Luke, Q. & Bytebier, B. (2017). Keetia namoyae (Rubiaceae, Vanguerieae), a new species from eastern Democratic Republic of Congo. Candollea 72: 23 – 26. https://doi.org/10.15553/c2017v721a2
Lovett, J. C. (1998). The Importance of the Eastern Arc Mountains for Vascular Plants. J. E. Africa Nat. Hist. Soc. Natl. Mus. 87: 59 – 74. https://doi.org/10.2982/0012-8317(1998)87[59:ioteam]2.0.co;2
Luke, W. R. Q., Musili, P., Barasa, J., Kalema, J. & Mathenge, J. (2018). Cola octoloboides. The IUCN Red List of Threatened Species 2018: e.T32257A111448374. https://doi.org/10.2305/IUCN.UK.2018-2.RLTS.T32257A111448374.en [Downloaded 9 May 2021].
Marshall, A. R., Couvreur, T. L. P., Summers, A. L., Deere, N. J., Luke, W. R. Q., Ndangalasi, H. J., Sparrow, S. & Johnson, D. M. (2016). A new species in the tree genus Polyceratocarpus (Annonaceae) from the Udzungwa Mountains of Tanzania. PhytoKeys 63: 63 – 76. https://doi.org/10.3897/phytokeys.63.6262
Moxon-Holt, L. & Cheek, M. (2021). Pseudohydrosme bogneri sp. nov. (Araceae), a spectacular Critically Endangered (Possibly Extinct) species from Gabon, long confused with Anchomanes nigritianus. BioRxiv. https://doi.org/10.1101/2021.03.25.437040
Ngumbau, V. M., Luke, Q., Nyange, M., Wanga, V. O., Watuma, B. M., Mbuni, Y. M., Munyao, J. N., Oulo, M. A., Mkala, E. M., Kipkoech, S., Itambo, M., Hu, G-W. & Wang, Q-F. (2020). An annotated checklist of the coastal forests of Kenya, East Africa. PhytoKeys 147: 1 – 191. https://phytokeys.pensoft.net/article/49602/
Nic Lughadha, E., Bachman, S. P. & Govaerts, R. (2017). Plant States and Fates: Response to Pimm & Raven. Trends Ecol. Evol. 32: 887 – 889. https://doi.org/10.1016/j.tree.2017.09.005
____, Govaerts, R., Belyaeva, I., Black, N., Lindon, H., Allkin, R., Magill, R. E. & Nicolson, N. (2016). Counting counts: revised estimates of numbers of accepted species of flowering plants, seed plants, vascular plants and land plants with a review of other recent estimates. Phytotaxa 272: 82 – 88. https://doi.org/10.11646/phytotaxa.272.1.5
Polhill, D. (1988). Index of Collecting localities. Flora of Tropical East Africa. Royal Botanic Gardens, Kew.
____ & Polhill, R. (2015). East African Plant Collectors. Kew Publishing. Royal Botanic Gardens, Kew.
Skarbek, C. (2008). A Review of Endemic Species in the Eastern Arc Afromontane Region: Importance, Inferences, and Conservation. Macalester Reviews in Biogeography: Vol. 1, Article 3. Available at http://digitalcommons.macalester.edu/biogeography/vol1/iss1/3
Soltis, D. E., Clayton, J. W., Davis, C. C., Wurdack, K. J., Gitzendanner, M. A., Cheek, M., Savolainen, V., Amorim, A. M. & Soltis, P. S. (2007). Monophyly and relationships of the enigmatic family Peridiscaceae. Taxon 56: 65 – 73.
Tanzania Forest Conservation Group (undated). http://www.tfcg.org/where-we-work/eastern-arc-mountains/ [Accessed 21 April 2021].
Thiers, B. (continuously updated). Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden's Virtual Herbarium. http://sweetgum.nybg.org/ih/ [Accessed May 2021].
Utteridge, T. M. A. (2021). Annonaceae. In: T. M. A. Utteridge & L. V. S. Jennings (eds), Trees of New Guinea, pp. 59 – 71. Royal Botanic Gardens, Kew.
Van Heusden, E. (1992). Flowers of Annonaceae. Blumea suppl. 7: 1 – 218.
Verdcourt, B. (1971). Annonaceae. In: E. Milne-Redhead & R. M. Polhill (eds), Flora of Tropical East Africa. Crown Agents, London.
____ (1986). New taxa of East African Annonaceae. Kew Bull. 41: 287 – 297. https://doi.org/10.2307/4102932
____ & Mwasumbi, L. B. (1988). A new species of Uvaria (Annonaceae) from Tanzania. Kew Bull. 43: 99 – 101. https://doi.org/10.2307/4118038
Vollesen, K. (1980). Notes on Annonaceae from Tanzania. Bot. Not. 133: 53 – 62.
Acknowledgements
We thank Dr Kaj Vollesen for making available to us the material published in this paper as Lukea, which he had been first to identify as a new genus. We thank Nina Davies for facilitating this. Janis Shillito typed the manuscript. Thomas Couvreur and Leo-Paul Dagallier of University of Montpellier are thanked for discussion, accelerating completion of this paper and Leo-Paul for sharing the results of his molecular phylogenetic research on the Uvariopsis clade. We thank Lars Chatrou and an anonymous reviewer for comments on an earlier version of this paper. Quentin Luke thanks Anthony Githitho of the National Museums of Kenya, Nairobi, and Base Titanium, of Kenya for supporting his visit to Kaya Ribe in May 2021.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to changes in the location of one of the isotypes.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cheek, M., Luke, W.R.Q. & Gosline, G. Lukea gen. nov. (Monodoreae-Annonaceae) with two new threatened species of shrub from the forests of the Udzungwas, Tanzania and Kaya Ribe, Kenya. Kew Bull 77, 647–664 (2022). https://doi.org/10.1007/s12225-022-10039-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12225-022-10039-y