Abstract
Purpose
A variety of lignocellulosic raw materials have been previously reported for the production of cellulose and cellulose derivatives, but little research effort has been dedicated to producing cellulose from Hyparrhenia filipendula. In this study, cellulose nanofibers (CNFs) were extracted from Hyparrhenia filipendula waste straws via sulphuric acid hydrolysis.
Methods
The straws were pretreated with a combination of physiochemical processes and hydrolyzed using sulphuric acid at three different concentrations (1 M, 3 M and 5.6 M) for 2 h at 80 °C. The properties of the CNFs was checked by Fourier Transform Infrared spectroscopy (FTIR) for surface chemistry, X-ray diffraction (XRD) for crystallinity, Scanning Electron microscopy and Transmission electron microscopy (TEM) for morphology. A high-performance liquid chromatograph (HPLC) was used to quantify the amount of biopolymers in the CNFs.
Results
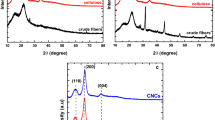
The results show that CNFs, denoted as CNF 1, CNF 3, and CNF 5.6 for 1 M, 3 M, and 5.6 M sulphuric acid, respectively, were successfully extracted at the various concentrations of sulphuric acid. The cellulose content of CNF1, CNF3, and CNF5.6 determined by HPLC analysis were 85%, 77% and 78% respectively. Also, the hemicellulose content in CNF 1, CNF 3, and CNF 5.6 was 10%, 15%, and 12% respectively, showing a high carbohydrate content of the CNFs. The FTIR spectra confirm the absence of characteristic peaks for lignin in the CNFs. The XRD analysis reveals presence of characteristic cellulose Iβ peaks at 2θ of 18°, 26°, and 40° with the crystallinity of 78%, 74% and 73% for CNF1, CNF3 and CNF5.6, respectively. Moreover, SEM analysis shows the deposition of lignin polycondensate on the surface of CNF 1, CNF 3, and CNF 5.6 while the bleached sample has a smooth and glossy appearance. The TEM analysis shows long unbranched nano-sized fibers for CNF 1 and shorter fibrous network of fibers for CNF 3, and CNF 5.6. The average diameter of the fibers, measured with ImageJ software is 40 nm for CNF 1, 57 nm for CNF 3, and 92 nm for CNF 5.6.
Conclusion
CNFs were successfully produced from Hyparrhenia filipendula and reported for the first time in open literature. In view of the structure and properties of the produced CNFs, they are a potential material for value-added applications such as polymer matrices, films, and membranes, thus enabling efficient utilization of agricultural waste.
Graphical Abstract






Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the study are not publicly available due to Copyright regulations by Makerere University and University of Pretoria, but are available from the corresponding author on reasonable request.
Abbreviations
- CNF:
-
Cellulose nanofiber
- FTIR:
-
Fourier transform infrared spectroscopy
- HPLC:
-
High-performance liquid chromatograph
- SEM:
-
Scanning electron microscopy
- TEM:
-
Transmission electron microscopy
- XRD:
-
X-ray powder diffraction
- °C:
-
Degrees centigrade
- h:
-
Hours
- nm:
-
Nanometers
References
Hachaichi, A., Kouini, B., Kian, L.K., Asim, M., Fouad, H., Jawaid, M., Sain, M.: Nanocrystalline cellulose from microcrystalline cellulose of date palm fibers as a promising candidate for bio-nanocomposites: isolation and characterization. Materials 14(18), 5313 (2021)
Moohan, J., Stewart, S.A., Espinosa, E., Rosal, A., Rodríguez, A., Larrañeta, E., Donnelly, R.F., Domínguez-Robles, J.: Cellulose nanofibers and other biopolymers for biomedical applications. A review. Appl. sci. 10(1), 65 (2019)
Yang, W., Feng, Y., He, H., Yang, Z.: Environmentally-friendly extraction of cellulose nanofibers from steam-explosion pretreated sugar beet pulp. Materials 11(7), 1160 (2018)
Djafari Petroudy, S.R., Chabot, B., Loranger, E., Naebe, M., Shojaeiarani, J., Gharehkhani, S., Ahvazi, B., Hu, J., Thomas, S.: Recent advances in cellulose nanofibers preparation through energy-efficient approaches: a review. Energies 14(20), 6792 (2021)
Trache, D., Tarchoun, A.F., Derradji, M., Hamidon, T.S., Masruchin, N., Brosse, N., Hussin, M.H.: Nanocellulose: from fundamentals to advanced applications. Front. Chem. 8, 392 (2020)
Bhat, A., Khan, I., Usmani, M.A., Umapathi, R., Al-Kindy, S.M.: Cellulose an ageless renewable green nanomaterial for medical applications: an overview of ionic liquids in extraction, separation and dissolution of cellulose. Int. J. Biol. Macromol. 129, 750–777 (2019)
Menon, M.P., Selvakumar, R., Ramakrishna, S.: Extraction and modification of cellulose nanofibers derived from biomass for environmental application. RSC Adv. 7(68), 42750–42773 (2017)
Pradhan, D., Jaiswal, A.K., Jaiswal, S.: Emerging technologies for the production of nanocellulose from lignocellulosic biomass. Carbohydr. Polym. (2022). https://doi.org/10.1016/j.carbpol.2022.119258
Nurul Atiqah, M.S., Gopakumar, D.A., Owolabi, F.A.T., Pottathara, Y.B., Samsul Rizal, N.A., Sri Aprilia, D., Hermawan, M.T., Paridah, S.T., Abdul, K.H., P. S,: Extraction of cellulose nanofibers via eco-friendly supercritical carbon dioxide treatment followed by mild acid hydrolysis and the fabrication of cellulose nanopapers. Polymers 11(11), 1813 (2019)
Tshivhase, V.M., Njinga, R.L., Mathuthu, M., Dlamini, T.: Transfer rates of 238U and 232Th for E. globulus, A. mearnsii, H. filipendula and hazardous effects of the usage of medicinal plants from around gold mine dump Environs. Int. J. Environ. Res. Public Health 12(12), 15782–15793 (2015)
Mbanyele, V., Mtambanengwe, F., Nezomba, H., Groot, J.C., Mapfumo, P.: Combinations of in-field moisture conservation and soil fertility management reduce effect of intra-seasonal dry spells on maize under semi-arid conditions. Field Crop Res 270, 108218 (2021)
Nagarajan, K., Ramanujam, N., Sanjay, M., Siengchin, S., Surya Rajan, B., Sathick Basha, K., Madhu, P., Raghav, G.: A comprehensive review on cellulose nanocrystals and cellulose nanofibers: Pretreatment, preparation, and characterization. Polym. Compos. 42(4), 1588–1630 (2021)
Dhali, K., Ghasemlou, M., Daver, F., Cass, P., Adhikari, B.: A review of nanocellulose as a new material towards environmental sustainability. Sci. Total Environ. 775, 145871 (2021)
Phanthong, P., Reubroycharoen, P., Hao, X., Xu, G., Abudula, A., Guan, G.: Nanocellulose: extraction and application. Carbon Resour. Convers 1(1), 32–43 (2018)
Hamawand, I., Seneweera, S., Kumarasinghe, P., Bundschuh, J.: Nanoparticle technology for separation of cellulose, hemicellulose and lignin nanoparticles from lignocellulose biomass: a short review. Nano-Struct. Nano-Objects 24, 100601 (2020)
Hazwan Hussin, M., Trache, D., Chuin, C.T.H., Nurul Fazita, M., Mohamad Haafiz, M., Hossain, M.: Extraction of cellulose nanofibers and their eco-friendly polymer composites, pp. 653–691. Springer, Berlin, Sustainable polymer composites and nanocomposites (2019)
Radakisnin, R., Abdul Majid, M.S., Jamir, M.R.M., Jawaid, M., Sultan, M.T.H., Mat Tahir, M.F.: Structural, morphological and thermal properties of cellulose nanofibers from Napier fiber (Pennisetum purpureum). Materials 13(18), 4125 (2020)
Khan, M.I., Lee, M.G., Shin, J.H., Kim, J.D.: Pretreatment optimization of the biomass of Microcystis aeruginosa for efficient bioethanol production. AMB Express 7(1), 1–9 (2017)
Adeeyo Opeyemi, A., Ayeni Augustine, O., Oladimeji Temitayo, E.: Acid hydrolysis of lignocellulosic content of sawdust to fermentable sugars for ethanol production. Int. J. Sci. Eng. Res. 6(3), 890–898 (2015)
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D., Crocker, D.: Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 1617(1), 1–16 (2008)
Mukwaya, V., Yu, W., Asad, R.A., Yagoub, H.: An environmentally friendly method for the isolation of cellulose nano fibrils from banana rachis fibers. Text. Res. J. 87(1), 81–90 (2017)
Waliszewska, B., Grzelak, M., Gaweł, E., Spek-Dźwigała, A., Sieradzka, A., Czekała, W.J.E.: Chemical characteristics of selected grass species from polish meadows and their potential utilization for energy generation purposes. Energies 14(6), 1669 (2021)
Doczekalska, B., Bartkowiak, M., Waliszewska, B., Orszulak, G., Cerazy-Waliszewska, J., Pniewski, T.: Characterization of chemically activated carbons prepared from miscanthus and switchgrass biomass. Materials 13(7), 1654 (2020)
Gou, G., Wei, W., Jiang, M., Zhang, S., Lu, T., Xie, X., Meng, F., Zhou, Z.: Environmentally friendly method for the separation of cellulose from steam-exploded rice straw and its high-value applications. In: Pulp and Paper Processing. In Tech (2018). https://doi.org/10.5772/intechopen.79014
Saeed, H., Liu, Y., Chen, H.: Exploring Sudanese agricultural residues as alternative fibres for pulp and paper manufacturing. IOP Conf. Ser. Mater. Sci. Eng. (2018). https://doi.org/10.1088/1757-899X/368/1/012030
Sluiter, A., Ruiz, R., Scarlata, C., Sluiter, J., Templeton, D.: Determination of extractives in biomass: laboratory analytical procedure (LAP). Natl. Renew. Energy Lab. 1617(1), 1–16 (2008)
Abraham, E., Deepa, B., Pothan, L.A., Jacob, M., Thomas, S., Cvelbar, U., Anandjiwala, R.: Extraction of nanocellulose fibrils from lignocellulosic fibres: A novel approach. Carbohyd. Polym. 86(4), 1468–1475 (2011)
Morcillo-Martín, R., Espinosa, E., Rabasco-Vílchez, L., Sanchez, L.M., de Haro, J., Rodríguez, A.: cellulose nanofiber-based aerogels from wheat straw: Influence of surface load and lignin content on their properties and dye removal capacity. Biomolecules 12(2), 232 (2022)
Wu, X.-Q., Liu, P.-D., Liu, Q., Xu, S.-Y., Zhang, Y.-C., Xu, W.-R., Liu, G.-D.: Production of cellulose nanofibrils and films from elephant grass using deep eutectic solvents and a solid acid catalyst. RSC Adv. 11(23), 14071–14078 (2021)
Pennells, J., Lin, T.Y., Schmidt, S., Gamage, H., Godwin, I.D., Erickson, T.E., Hosseinmardi, A., Martin, D.J., Amiralian, N.: Effects of the growth environment on the yield and material properties of nanocellulose derived from the Australian desert grass Triodia. Ind. Crops Prod. 126, 238–249 (2018)
Wang, C., Li, H., Li, M., Bian, J., Sun, R.: Revealing the structure and distribution changes of Eucalyptus lignin during the hydrothermal and alkaline pretreatments. Sci. Rep. 7(1), 1–10 (2017)
Zhu, W., Zhang, Y., Wang, X., Wu, Y., Han, M., You, J., Jia, C., Kim, J.: Aerogel nanoarchitectonics based on cellulose nanocrystals and nanofibers from eucalyptus pulp: preparation and comparative study. Cellulose 29(2), 817–833 (2022)
Jebali, Z., Nabili, A., Majdoub, H., Boufi, S.: Cellulose nanofibrils (CNFs) from Ammophila arenaria, a natural and a fast growing grass plant. Int. J. Biol. Macromol. 107, 530–536 (2018)
Klančnik, K., Vogel-Mikuš, K., Gaberščik, A.: Silicified structures affect leaf optical properties in grasses and sedge. J. Photochem. Photobiol., B 130, 1–10 (2014)
Qadi, N., Takeno, K., Mosqueda, A., Kobayashi, M., Motoyama, Y., Yoshikawa, K.: Effect of hydrothermal carbonization conditions on the physicochemical properties and gasification reactivity of energy grass. Energy Fuels 33(7), 6436–6443 (2019)
Essien, E.A., Kavaz, D.: Effective and reusable nano-silica synthesized from barley and wheat grass for the removal of nickel from agricultural wastewater. Environ. Sci. Pollut. Res. 26(25), 25802–25813 (2019)
Zang, S., Zuo, Y., Wang, J., Liu, X., Gomez, M.A., Wei, L.: Adsorption removal of roxarsone, arsenite (III), and arsenate (V) using iron-modified sorghum straw biochar and its kinetics. Acta Geochimica 40(3), 409–418 (2021)
Nagarajan, K., Balaji, A., Ramanujam, N.: Extraction of cellulose nanofibers from cocos nucifera var aurantiaca peduncle by ball milling combined with chemical treatment. Carbohydr. Polym. 212, 312–322 (2019)
Zhang, H., Chen, Y., Wang, S., Ma, L., Yu, Y., Dai, H., Zhang, Y.J.C.P.: Extraction and comparison of cellulose nanocrystals from lemon (Citrus limon) seeds using sulfuric acid hydrolysis and oxidation methods. Carbohydr. Polym. (2020). https://doi.org/10.1016/j.carbpol.2020.116180
Głazowska, S., Baldwin, L., Mravec, J., Bukh, C., Hansen, T.H., Jensen, M.M., Fangel, J.U., Willats, W.G., Glasius, M., Felby, C.: The impact of silicon on cell wall composition and enzymatic saccharification of Brachypodium distachyon. Biotechnol. Biofuels 11(1), 1–18 (2018)
Kumari, P., Pathak, G., Gupta, R., Sharma, D., Meena, A.: Cellulose nanofibers from lignocellulosic biomass of lemongrass using enzymatic hydrolysis: characterization and cytotoxicity assessment. DARU J. Pharm. Sci. 27(2), 683–693 (2019)
Sánchez-Gutiérrez, M., Espinosa, E., Bascón-Villegas, I., Pérez-Rodríguez, F., Carrasco, E., Rodríguez, A.: Production of cellulose nanofibers from olive tree harvest—A residue with wide applications. Agronomy 10(5), 696 (2020)
Wang, J., Wang, Q., Wu, Y., Bai, F., Wang, H., Si, S., Lu, Y., Li, X., Wang, S.: Preparation of cellulose nanofibers from bagasse by phosphoric acid and hydrogen peroxide enables fibrillation via a swelling, hydrolysis, and oxidation cooperative mechanism. Nanomaterials 10(11), 2227 (2020)
Funding
This work was supported by the African Center of Excellence in Materials, Product Development and Nanotechnology (MAPRONANO ACE) funded by the World Bank and Government of Uganda [Project Identification P151847, IDA Number 5797-UG]. Ayaa Fildah also acknowledges support received from The Prof. Daramola Development Fund that enabled her to visit and supervise this study at the University of Pretoria. Ndwandwa Nolundi acknowledges the Chemical Industries Education & Training Authority (CHIETA) of South Africa for funding her internship at the University of Pretoria allowing her to complete this study under the supervision of Prof. Daramola and Ayaa Fildah.
Author information
Authors and Affiliations
Contributions
All authors contributed to the successful completion of this study. AF, SAI, MOD, and JBK conceptualized the project and reviewed the first draft. MOD, Samuel AI and JBK were responsible for supervision, funding acquisition and project administration.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ndwandwa, N., Ayaa, F., Iwarere, S.A. et al. Extraction and Characterization of Cellulose Nanofibers From Yellow Thatching Grass (Hyparrhenia filipendula) Straws via Acid Hydrolysis. Waste Biomass Valor 14, 2599–2608 (2023). https://doi.org/10.1007/s12649-022-02014-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-022-02014-2