Photo credit: Google
Habenaria marginata (Golden Yellow Habenaria)
Stomatal ontogeny in Habenaria marginata Coleb.
by Inamdar J. A. (1968)
Sardar Patel University, Vallabh Vidyanagar, Gujarat, India
in Curr. Sci. 37: 24-25

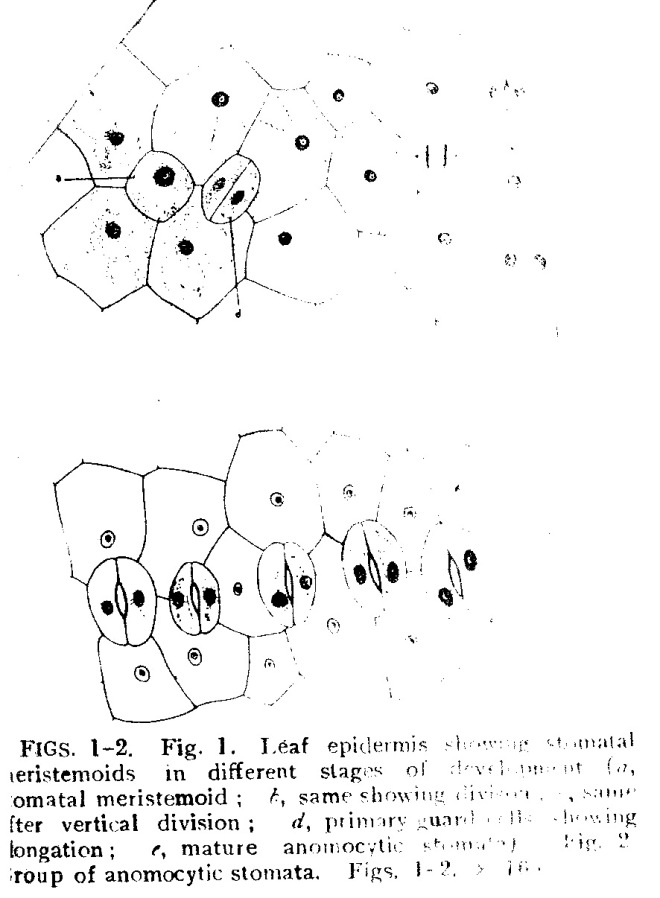
Photo credit: Google
Habenaria marginata (Golden Yellow Habenaria)
by Inamdar J. A. (1968)
Sardar Patel University, Vallabh Vidyanagar, Gujarat, India
in Curr. Sci. 37: 24-25


Photo credit: Google
Heirloom Fava bean Broad Windsor Seeds
by Talbott L. D., Zeiger E. (1996)
L. D. Talbott
Eduardo Zeiger
in Plant Physiol. 111, 1051–1057 -PMID: 12226347 PMCID: PMC160980 – DOI: https://doi.org/10.1104/pp.111.4.1051
PubMed Abstract | Google Scholar –
http://www.plantphysiol.org/content/111/4/1051.short
Abstract
Osmoregulation in guard cells of intact, attached Vicia faba leaves grown under growth chamber and greenhouse conditions was studied over a daily light cycle of stomatal movements.
Under both growth conditions guard cells had two distinct osmoregulatory phases. In the first (morning) phase, opening was correlated with K+ uptake and, to a lesser extent, sucrose accumulation. In the second (afternoon) phase, in which apertures were maximal, K+ content declined and sucrose became the dominant osmoticum.
Reopening of the stomata after a CO2-induced closure was accompanied by accumulation of either K+ or sucrose, depending on the time of day, indicating that a single environmental signal may use multiple osmoregulatory pathways.
Malate accumulation, correlated with K+ uptake, was detected under growth chamber but not greenhouse conditions, whereas Cl- was the main K+ counterion in the greenhouse.
These results indicate that guard-cell osmoregulation in the intact leaf depends on at least two different osmoregulatory pathways, K+ transport and sucrose metabolism. Furthermore, the relative importance of the K+ counterions malate and Cl- appears to be environment-dependent.
Photo credit: Google
by Tallman G., Zeiger E. (1988)
,
– Plant and Cell Physiology 1988;88:887-895 – DOI: https://doi.org/10.1104/pp.88.3.887 –
Google Scholar – CrossRef |PubMed | –
http://www.plantphysiol.org/content/88/3/887
Abstract
Osmoregulation in opening stomata of epidermal peels from Vicia faba L. leaves was investigated under a variety of experimental conditions. The K+content of stomatal guard cells and the starch content of guard cell chloroplasts were examined with cobaltinitrite and iodine-potassium iodide stains, respectively; stomatal apertures were measured microscopically.
Red light (50 micromoles per square meter per second) irradiation caused a net increase of 3.1 micrometers in aperture and a decrease of −0.4 megapascals in guard cell osmotic potential over a 5 hour incubation, but histochemical observations showed no increase in guard cell K+ content or starch degradation in guard cell chloroplasts.
At 10 micromoles per square meter per second, blue light caused a net 6.8 micrometer increase in aperture over 5 hours and there was a substantial decrease in starch content of chloroplasts but no increase in guard cell K+ content.
At 25 micromoles per square meter per second of blue light, apertures increased faster (net gain of 5.7 micrometers after 1 hour) and starch content decreased. About 80% of guard cells had a higher K+ content after 1 hour of incubation but that fraction decreased to 10% after 5 hours.
In the absence of KCl in the incubation medium, stomata opened slowly in response to 25 micomoles per square meter per second of blue light, without any K+ gain or starch loss.
In dual beam experiments, stomata irradiated with 50 micomoles per square meter per second of red light for 3 hours opened without detectable starch loss or K+ gain; addition of 25 micomoles per square meter per second of blue light caused a further net gain of 4.4 micometers in aperture accompanied by substantial K+ uptake and starch loss.
Comparison of K+ content in guard cells of opened stomata in epidermal peels with those induced to open in leaf discs showed a substantially higher K+ content in the intact tissue than in isolated peels.
These results are not consistent with K+ (and its counterions) as the universal osmoticum in guard cells of open stomata under all conditions; rather, the data point to sugars arising from photosynthesis and from starch degradation as additional osmotica.
Biochemical confirmation of these findings would indicate that osmoregulation during stomatal opening is the result of three key metabolic processes: ion transport, photosynthesis, and sugar metabolism.
Photo credit: Google
Sunflower Seeds Field in bloom .
by Tardieu F., Lafarge T., Simonneau T. H. (1996)
F. Tardieu, INRA, Ecophysiologie des Plantes sous Stress Environmentaux (LEPSE). 34060 Montpellier cedex l, France.
in Plant Cell Environ. 1996;19:75–84 – –
http://onlinelibrary.wiley.com/doi/10.1111/j.1365-3040.1996.tb00228.x/full
ABSTRACT
The stomatal conductance of several anisohydric plant species, including field-grown sunflower, frequently correlates with leaf water potential (φ1), suggesting that chemical messages travelling from roots to shoots may not play an important role in stomatal control.
We have performed a series of experiments in which evaporative demand, soil water status and ABA origin (endogenous or artificial) were varied in order to analyse stomatal control. Sunflower plants were subjected to a range of soil water potentials under contrasting air vapour pressure deficits (VPD, from 0.5 to 2.5 kPa) in the field, in the glasshouse or in a humid chamber. Sunflower plants were also fed through the xylem with varying concentrations of artificial ABA, in the glasshouse and in the field. Finally, detached leaves were fed directly with varying concentrations of ABA under three contrasting VPDs.
A unique relationship between stomatal conductance (gs) and the concentration of ABA in the xylem sap (xylem [ABA]) was observed in all cases. In contrast, the relationship between φ1 and gs varied substantially among experiments. Its slope was positive for droughted plants and negative for ABA-fed whole plants or detached leaves, and also varied appreciably with air VPD. All observed relationships could be modelled on the basis of the assumption that φ1 had no controlling effect on gs.
We conclude that stomatal control depended only on the concentration of ABA in the xylem sap, and that φ1 was controlled by water flux through the plant (itself controlled by stomatal conductance).
The possibility is also raised that differences in stomatal ‘strategy’ between isohydric plants (such as maize, where daytime φ1 does not vary appreciably with soil water status) and anisohydric plants (such as sunflower) may be accounted for by the degree of influence of φ1 on stomatal control, for a given level of xylem [ABA]. We propose that statistical relationships between φ1 and gs are only observed when φ1 has no controlling action on stomatal behaviour.
Photo credit: Google
Yucca filamentosa
by Inamdar J. A., Gangadhara M., Bhat R. B. (1977)
Sardar Patel University, Vallabh Vidyanagar, Gujarat, India
in Ceylon J. Sci. 12(2): 119-124

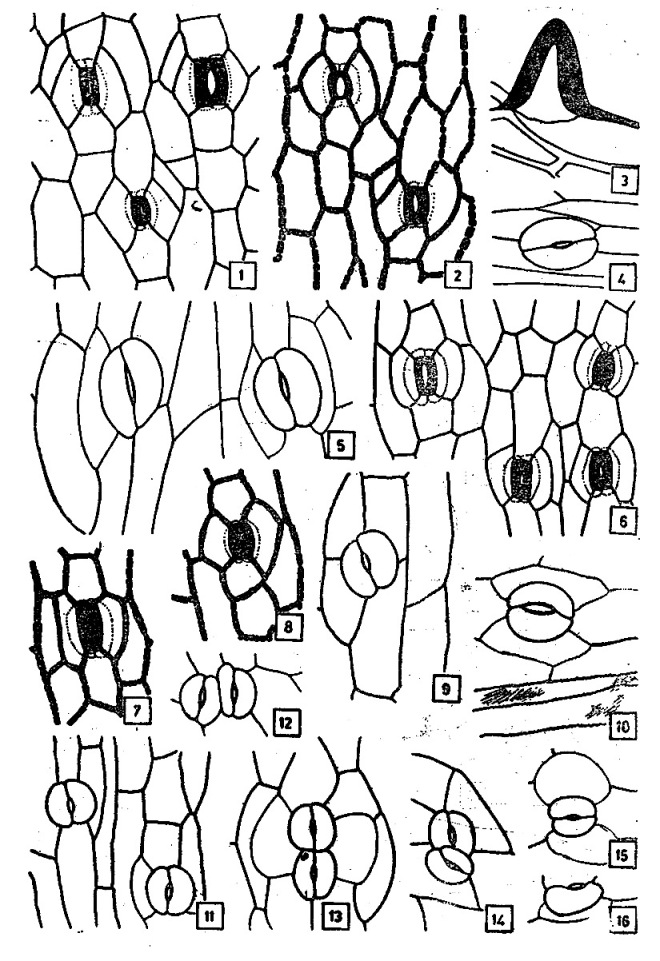


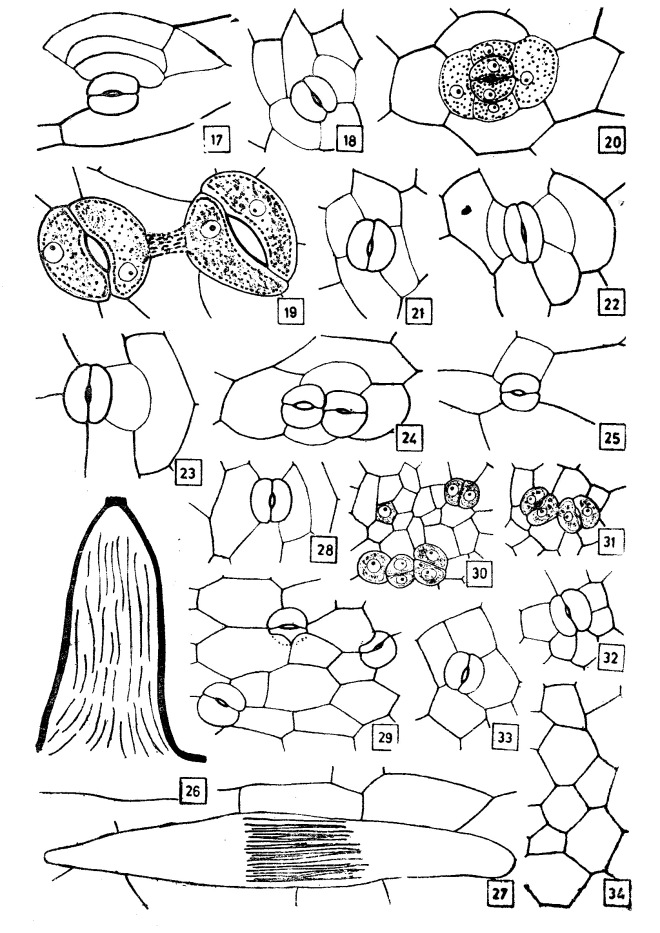
Photo credit: Google
Vahlia capensis var vulgaris
by Inamdar J. A., Patel R. C. (1971)
in Acta Bot. Acad. Sci. Hungar. 17)3-4): 361-369

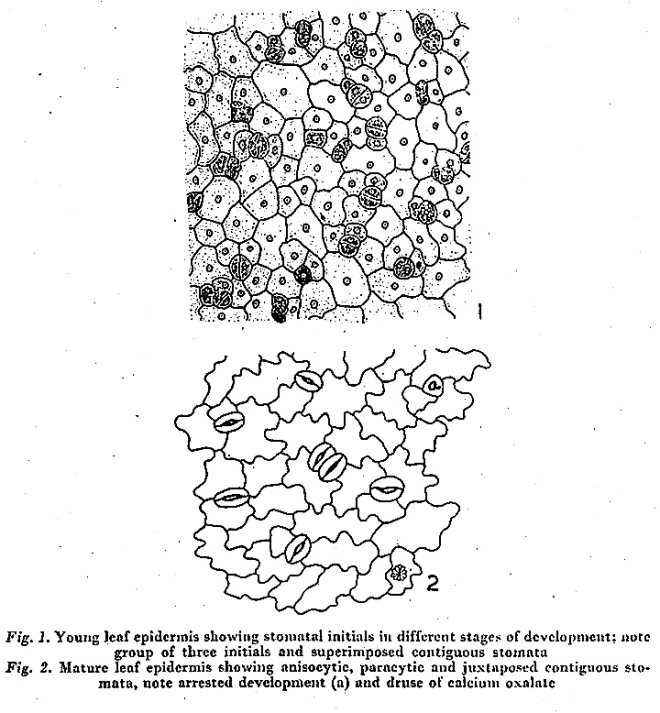
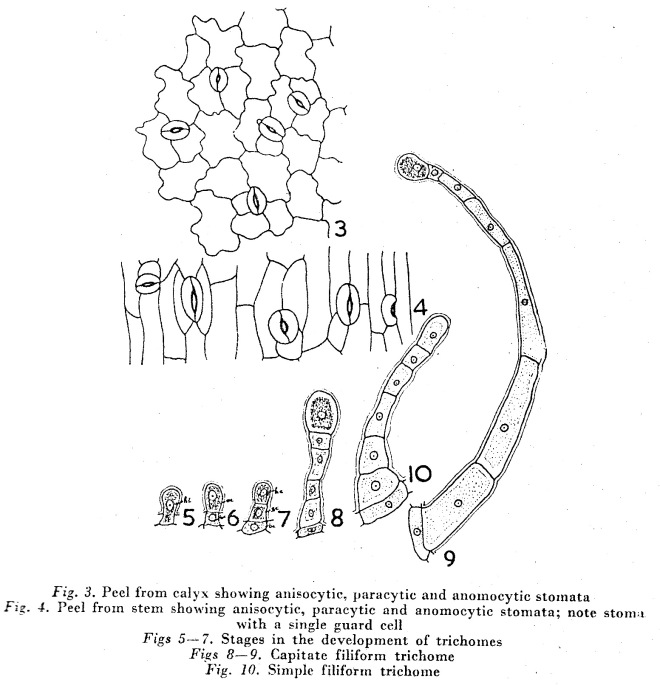
Photo credit: Google
Maize – Zea mays
, , , , , (1992)
in Plant, Cell & Environment 15: 193–197 – –
Wiley Online Library |PubMed |CAS | CrossRef Web of Science Google Scholar –
http://onlinelibrary.wiley.com/doi/10.1111/j.1365-3040.1992.tb01473.x/full
ABSTRACT
Stomatal conductance of individual leaves was measured in a maize field, together with leaf water potential, leaf turgor, xylem ABA concentration and leaf ABA concentration in the same leaves.
Stomatal conductance showed a tight relationship with xylem ABA, but not with the current leaf water status or with the concentration of ABA in the bulk leaf.
The relationship between stomatal conductance and xylem [ABA] was common for variations in xylem [ABA] linked to the decline with time of the soil water reserve, to simultaneous differences between plants grown on compacted, non-compacted and irrigated soil, and to plant-to-plant variability. Therefore, this relationship is unlikely to be fortuitous or due to synchronous variations.
These results suggest that increased concentration of ABA in the xylem sap in response to stress can control the gas exchange of plants under field conditions.
Photo credit: Google
Tree Tobacco, Nicotiana glauca
by Taylor J. E., Abram B., Boorse G., Tallman G. (1998)
in Journal of Experimental Botany 49: 377–386 –DOI: 10.1093/jexbot/49.suppl_1.377 –
Guard cell protoplasts (GCP) of Nicotiana glauca (Graham), tree tobacco, were cultured at 32 degrees C or at 38 degrees C in media containing or lacking 0.1 mu M ABA. Cells cultured at 32 degrees C exhibited large increases in cell volume, dedifferentiated, and divided (Type II cells). Cells cultured at 38 degrees C increased less in volume, retained the general morphology of guard cells, and did not divide (Type III cells). Cells cultured at 38 degrees C in media containing ABA (Type IV cells) neither grew nor divided; they retained the morphology of freshly-isolated GCP (FGCP).
Experiments were performed to determine the extent to which selected cell types retained certain physiological, molecular characteristics of FGCP. increased in volume by similar to 40% when illuminated with low-intensity blue light (15 mu mol m(-2) s(-1)) over background red light (300 mu mol m(-2) s(-1)). Blue light-induced swelling of GCP was inhibited fully by 10 mu M m-chlorophenylhydrazone and 5 mM dithiothreitol. Chloroplasts of Type II cells underwent senescence.
The capacity of chloroplasts of Type III and Type IV cells for photochemical quenching of Chi a fluorescence was reduced compared to that of FGCP, and cultured cells lost all capacity for non-photochemical quenching.
Zeaxanthin (Z) has been identified as the putative blue light photoreceptor of guard cells. Type III cells lost capacity for light-induced Z formation, but Type IV cells retained the capacity to form Z. Results of differential display-PCR indicated that the greatest number of absolute differences in PCR products was between FGCP and Type II cells; the fewest number was between FGCP and Type IV cells. Both the blue light photoreceptor and the signal transduction pathway linking blue light photoreception to activation of the plasma membrane H+-translocating ATPase of guard cells remain intact in cultured Type IV cells.
Culture conditions alter both photochemical and nonphotochemical processes of chloroplasts of Type III and Type IV cells, but chloroplasts of Type IV cells retain the capacity to acidify the thylakoid lumen to activate a functional violaxanthin-antheraxanthin de-epoxidase in the thylakoid membrane.
The data support the hypothesis that each cell type exists in a differentiated state more or less similar to that of FGCP, with Type II cells having the least similarity, Type III cells having greater similarity, and Type IV cells having the greatest similarity.
Photo credit: Google
by Thakur P. S., Thakur A., Rai V. K. (1988)
Department of Bio-Sciences, H. P. University Shimla, India
in Biochemie und Physiologie der Pflanzen, 1988.183 (1), 37 – 43 – https://doi.org/10.1016/S0015-3796(88)80066-0 –
CrossRef. –
http://www.sciencedirect.com/science/article/pii/S0015379688800660?via%3Dihub
Summary
Exogenously applied L-alanine, L-glutamic acid, L-lysine, L-proline, L-arginine and L-methionine, all promoted stomatal opening in Commelina communis L. epidermal peels.
All of these aminoacids tested, irrespective of their individual effectiveness antagonized the closure of stomata induced either by different stress levels or abscisic acid.
Stomata responded variably to imposed stress where aperture size declined from 19.7 µm in control to 7.6, µrn by – 0.808 MPa upto 6 h of treatment. The closure was even more at higher osmotic potential (i.e. −1.10 — −1.61 MPa). ABA (between 10-9 to 10-5 mol m-3) reduced stomatal aperture significantly.
The relevance of amino acids in relation to stomatal aperture is discussed.
Photo credit: Google
Oat seedlings
by Thimann K. V., Satler S. O. (1979)
in Current Issue 76 no. 5 , 2295–2298 –
http://www.pnas.org/content/76/5/2295
Abstract
Senescence of isolated oat seedling leaves, floating on water or solutions in white light, has been followed by the disappearance of chlorophyll and the liberation of free amino nitrogen. In parallel, measurements of stomatal aperture were made with a diffusion resistance porometer, and borne out also by changes in fresh weight.
The stomata open as expected in the light but slowly begin to close after the first day; correspondingly, in the dark they close at once but gradually begin to open on successive days.
Abscisic acid causes closure and this is accompanied by senescence. Phenylmercuric nitrate also causes closure and again the reaction is closely paralleled by senescence.
Kinetin maintains stomatal opening even more than does light alone, and this is accompanied by complete prevention of chlorophyll loss for at least 5 days. Covering the leaf surface with a film of Vaseline, especially when detergents are added, accelerates senescence.
Merely floating the leaf segments on hypertonic solutions of sucrose or mannitol suffices to bring the rate of senescence in light up to the rate in darkness.
It is concluded that the effect of light in delaying senescence is primarily due to its effect on the stomatal aperture, and, more generally, that stomatal aperture is the principal controlling agent in leaf senescence.
You must be logged in to post a comment.