Critical Insights Into the Ecological and Invasive Attributes of Leucaena leucocephala, a Tropical Agroforestry Species
- 1Department of Environment Studies, Panjab University, Chandigarh, India
- 2Department of Botany, Panjab University, Chandigarh, India
- 3Queensland Alliance for Agriculture and Food Innovation (QAAFI), School of Agriculture and Food Sciences (SAFS), The University of Queensland, St Lucia, QLD, Australia
- 4Department of Agronomy, Chaudhary Charan Singh Haryana Agricultural University (CCSHAU), Hisar, India
Leucaena leucocephala (Lam.) de Wit (commonly known as leucaena) is a leguminous species of the family Fabaceae and a native of Mexico and Central America. It is often addressed as a “miracle tree” for offering a wide variety of ecosystem services and possessing strong ecological attributes. The multiple uses of leucaena in agroforestry, livestock, and restoration practices led to the worldwide distribution of its ssp. glabrata and leucocephala. However, following its introduction into non-native regions, the commercial value of ssp. leucocephala was challenged by its large-scale spread outside the cultivation zone. It has assumed a status of an environmental weed and invasive plant in many regions across Africa (17 countries and Island nations), Asia (17), Europe (1), Oceania (23), North America (12), and South America (7). The plant is enlisted in the top five terrestrial invasive plant species with the greatest international presence. The species is now considered one of the 100 worst invaders in the world. The plant mainly invades roadsides, wastelands, cultivated lands, riverbanks, and forest edges, and suppresses the growth of other woody and herbaceous species. Its infestations alter the patterns of vegetation, plant succession, and community assembly in the introduced habitats. Propagation of ssp. leucocephala, without considering the environmental risks associated with it, may result in major repercussions and irreparable losses. Therefore, it is important to discuss its invasive propensities and the possible alternatives that may replace the weedy species without encumbering its economic benefits. This review aims to thoroughly evaluate the ecological and invasive attributes of leucaena, promote awareness about the ecological costs associated with its spread, and suggest suitable options for its management.
Introduction
Leucaena leucocephala (Lam.) de Wit (hereafter, leucaena) is a leguminous species of the family Fabaceae (sub-family: Mimosoideae), indigenous to Mexico and Central America (Olckers, 2011; Batisteli et al., 2020). Three sub-species of leucaena are reported in the literature, namely L. leucocephala ssp. glabrata, L. leucocephala ssp. leucocephala, and L. leucocephala ssp. ixtahuacana. Leucaena is often addressed as a “miracle tree” for offering a wide variety of ecosystem services and possessing strong ecological attributes such as fast growth, quick regeneration, nitrogen-fixing ability, and tolerance toward nutrient-poor soils, high temperature, drought, and salinity (Chaturvedi and Jha, 1992; Chen et al., 2018; Bageel et al., 2020). These attributes contributed to its popularity in several national and international agroforestry programs (Brewbaker, 1987; Harris et al., 1994), livestock production and feeding systems (González-García et al., 2009), and restoration strategies for the degraded ecosystems (Chen et al., 2018), which led to its worldwide distribution (mainly ssp. glabrata and leucocephala) during the 19th and 20th century.
Although leucaena has been acclaimed as a multipurpose tree that has provided numerous economic benefits to the stakeholders and supported the livelihoods of several small-scale farmers; however, following its introduction, ssp. leucocephala has assumed the status of an environmental weed in most of its non-native regions (Olckers, 2011). It has been declared a troublesome invasive plant with severe ecological implications in several parts of Africa, Asia, Oceania, and South America (CABI, 2021). It is now considered one of the 100 worst invaders in the world (GISD, 2021). Several countries have restricted the plantations of ssp. leucocephala because of its adverse impacts on biodiversity, while others have continued propagating the species for different commercial and non-commercial purposes. Propagation of ssp. leucocephala, without considering the environmental risks associated with it, may result in major repercussions and irreparable losses. Therefore, it is important to discuss its invasive propensities and the possible alternatives that may replace the weedy species without encumbering its economic benefits. This review aims to thoroughly evaluate the ecological and invasive attributes of leucaena, promote awareness about the ecological costs associated with its spread, and suggest suitable options for its management.
Morphological Characteristics
Leucaena is an evergreen tree, often reaching a height of 7–18 m (Chiou et al., 2013; Figure 1). The root system of mature plants is deep, with an architectural composition of long taproots, fine tertiary roots, and numerous robust lateral roots (Pandey and Kumar, 2013). The stem is woody, cylindrical, branched, and dark brown (Chiou et al., 2013). Leaves are bipinnate and slightly asymmetric with 6–8 pairs of leaflets (Chiou et al., 2013; Figure 1). The inflorescence is usually borne on actively growing shoots, cream in color, and globular/capitate in shape with 100–180 flowers per head (Pandey and Kumar, 2013; Figure 1). Each flower head produces 5–25 pods that are flat, linear to oblong in shape, lustrous, covered with velvety hairs, and consists of 15–30 seeds, which are released from both margins of the pod (Hwang et al., 2010; Figure 1). Seeds are small (6.7–9.6 mm long, 4–6.3 mm wide, and 0.080–0.070 g in mass) with a flat surface, hard testa, discoidal shape, dark brown color, and transverse alignment in the pods (Pandey and Kumar, 2013; Ngongolo et al., 2014; Batisteli et al., 2020; Figure 1). Due to its rapid growth rate, it is estimated to have annual biomass productivity of 50 tons per hectare in the Mediterranean and tropical regions (Awang et al., 2019).

Figure 1. Physical characteristics of Leucaena leucocephala, (A) reproductive buds, (B) Inflorescence, (C,D) Flowering shoot, (E) Mature pods, (F) Seeds.
Subspecies, Types, and Hybridization
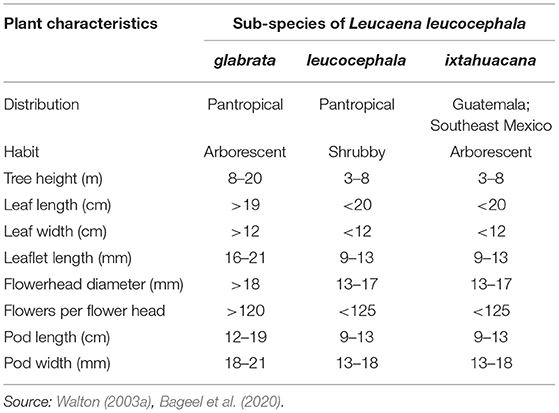
Three sub-species of leucaena, namely L. leucocephala ssp. glabrata, L. leucocephala ssp. leucocephala, and L. leucocephala ssp. ixtahuacana have been identified (Lok et al., 2010). Of these, ssp. leucocephala (also known as common leucaena) and glabrata (also known as giant leucaena) have been introduced pantropically and ssp. leucocephala is the most widely distributed. Compared to the large size of ssp. glabrata, ssp. leucocephala is a small tree or bushy shrub with huge reproductive capacity that resulted in its widespread naturalization, and therefore, it is considered an aggressive colonizer of ruderal sites, degraded lands, and occasionally agricultural fields (Bageel et al., 2020). Morphological variations in the three subspecies are listed in Table 1. Leucaena has also been differentiated into three distinct types, i.e., “Hawaiian” or “common” type, which is a short (5 m) and bushy variant; “Peru” type, which is a tall (15 m) and extensively branched variant; and “Salvador” or “Hawaiian Giant” type, which is a tall (20 m) and unbranched variant. The “Hawaiian” type refers to ssp. leucocephala, whereas the “Peru” and “Salvador” types are placed under ssp. glabrata (MacLaurin et al., 1981; ICAR, 2006).
Interspecific hybridization contributes significantly to the origin of new species, particularly in the case of polyploid species such as leucaena (Gaskin, 2017). Leucaena is an allotetraploid species, that evolved through the natural hybridization between L. pulverulenta and L. lanceolate (Bageel et al., 2020). Polyploid species usually can restore sexual reproduction after hybridization (Te Beest et al., 2012), and therefore, several fertile hybrids of leucaena have been reported. The most common hybridization has been observed between L. leucocephala ssp. leucocephala (“Hawaiian” type) and ssp. glabrata (“Peru” type) and the resultant hybrid is suggested to be the most suitable foraging variety (MacLaurin et al., 1981). Bageel et al. (2020) listed several promising hybrids of leucaena for cultivation like K5, K8, K28, K29, K67, K132, Cunningham, K584, Tarramba, K748, K1000, Redlands, Lanang, Wondergraze, etc., to obtain high biomass yields, digestibility, wood yield, seedling vigor, cold tolerance, and psyllid resistance. There are also several interspecific hybrids of leucaena that produce either no or very few seeds. Cultivar KX2-Hawaii (developed by selection from advanced generations of the original F1 hybrid L. pallida K376 × L. leucocephala ssp. glabrata K8) and Cultivar Redlands (developed using 5 elite KX2 F1 hybrids) are highly efficient but self-sterile hybrids of leucaena (Dalzell, 2019). Cultivar KX4-Hawaii is a male-sterile triploid hybrid between L. leucocephala ssp. glabrata K636 and L. esculenta K838, which is vegetatively propagated, psyllid-resistant, arboreal, vigorous, and cold-tolerant (Dalzell, 2019). The recently instigated research projects focusing on the development of absolutely sterile cultivars of leucaena may lead to a series of mutagenized seedless plants in the near future (Buck et al., 2019; McMillan et al., 2019).
Geographic Distribution and Ecological Requirements
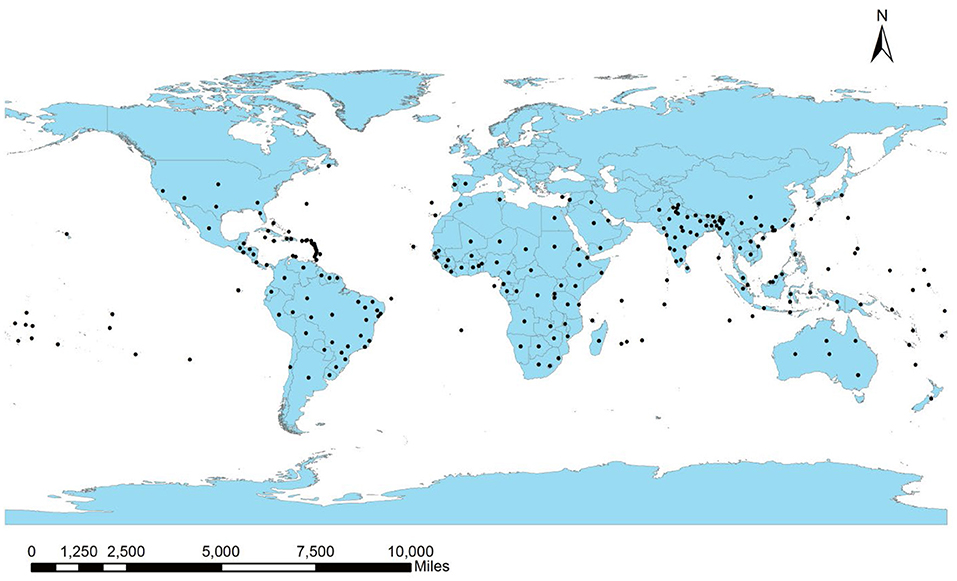
Leucaena is a native inhabitant of Mexico and Central America and has been introduced worldwide for its commercial value. Its distribution has been recorded within 15–25° (North or South) of the equator. The global distribution of the plant is presented in Figure 2, according to the database of CABI (2021). Marod et al. (2012) described the species' preference for sub-humid or humid conditions, a warm environment with a temperature >15°C, and annual precipitation ranging within 500–3,500 mm for germination and growth (Kodiango and Palapala, 2016). Leucaena favors alkaline and well-drained soils with pH from neutral to slightly basic and an altitude lower than 1,400 m (Chiou et al., 2013; Verdecia et al., 2020). It has been reported to be intolerant to frost and shade (Chiou et al., 2013). Also, available nitrogen and phosphorus are the major limitations for leucaena (Lin et al., 2019); however, the plant can fix nitrogen from the atmosphere via establishing symbiosis with nitrogen-fixing bacteria belonging to the genera, Mesorhizobium and Rhizobium (Sithole et al., 2021). Under adverse environmental conditions, the plant may develop several physiological and biochemical strategies to combat abiotic stress (da Silva Rodrigues-Corrêa et al., 2019).
Germination and Reproductive Biology
Epigeal germination is observed in the seeds of leucaena, which occurs with or without scarification (Parrotta, 1992). Seeds are reported to germinate in a temperature range of 15–40°C, and a pH range of 4–9. This justifies the preference of leucaena for warm climates and alkaline soils, although the germination ability of the plant in acidic environments has also been successfully demonstrated (Kodiango and Palapala, 2016). Seeds have a partial light requirement and can emerge from a burial depth of 0–5 cm, while increasing the burial depth decreases the seed viability in a directly proportional manner (Hwang et al., 2010). For plantation, 2–3 months old seedlings are suitable, which can be propagated through direct seeding, stem cuttings, and bare-root/container seedlings (Parrotta, 1992). The seed germination and growth rate of leucaena are limited in the colder regions (<15°C), acidic soils with high aluminum or calcium content, water-logging conditions, and under psyllid or termite attack (Timyan, 1996). Moreover, no germination was observed below the osmotic potential of −0.4 MPa (Hwang et al., 2010).
Leucaena is a self-compatible tetraploid (2n = 4x = 104); however, out-crossing has also been observed in the plant (Harris et al., 1994; Olckers, 2011). In the invaded ranges, flowering has been reported all year round, depending on the availability of adequate moisture (Wu et al., 2013; Marques et al., 2014). Parrotta (1992) reported the onset of flowering within 4–6 months of germination. Pollination is achieved through entomophily, primarily through bees, and seed set has been observed in one-year-old seedlings (Ngongolo et al., 2014). With the onset of fruiting and seed set, a corresponding suppression of vegetative growth has also been documented (Orwa et al., 2009). The pods ripen after 10–15 weeks of seed setting but under drought stress, the pods are retained for a longer time (Raghu et al., 2005). The plant usually has flowers and pods (immature and mature) simultaneously (Batisteli et al., 2020).
The pods usually release seeds while still being attached to the branch, although unopened or partially opened pods may also detach from the main plant and disperse along with the seeds through anemochory (Parrotta, 1992; Batisteli et al., 2020). Livestock or wild animals may also act as dispersal agents (zoochory) by consuming the pods and passing the undigested (or partially digested) seeds through their digestive tract (Parrotta, 1992). The seeds can remain viable for 10–20 years owing to their hard testa, thereby forming a persistent seed bank in the soil (Olckers, 2011). Leucaena can produce ~5,500 seeds m−2 per year (Marques et al., 2014). This large number of seeds forms a persistent seed bank of the plant with a viability of 1–5 years (Marques et al., 2014). Additionally, vegetative reproduction via resprouting of shoots has been documented after cutting and events of forest fire (Agriculture Victoria, 2021).
Mimosine: A Characteristic Secondary Metabolite of Leucaena
Mimosine is a non-proteinaceous amino acid and a secondary metabolite present in all plant parts of leucaena, with the maximum concentration being reported in young foliage (Honda and Borthakur, 2019). Several biological activities such as anticancer, antifungal, antimicrobial, defense against herbivores and pests, and allelopathy are associated with this molecule (da Silva Rodrigues-Corrêa et al., 2019). Moreover, mimosine is a phytosiderophore, secreted by the roots of leucaena into the rhizosphere for uptake of metallic cations such as Fe(III), Zn(II), Cu(II), and Mn(II), as a result of which leucaena can grow in calcareous alkaline soils where other plants cannot flourish (Honda and Borthakur, 2020). Recent studies also describe mimosine as a stress-response molecule involved in general oxidative stress modulation acting as a hydrogen peroxide and superoxide anion quencher (da Silva Rodrigues-Corrêa et al., 2019). In ssp. glabrata, it is produced in large quantities under favorable growth environments and utilized as a source of carbon and nitrogen under low-nutrient conditions (Honda and Borthakur, 2021). Most leucaena-nodulating rhizobia can degrade and utilize mimosine as a source of nutrients (Soedarjo and Borthakur, 1998).
On the contrary, mimosine is reported to be toxic to other plants and animals. Once ingested by animals (ruminants and non-ruminants), it is converted to 3-hydroxy-4(1H)-pyridone (3H4P) and 2,3-dihydroxypyridine (2,3-DHP) (Ilham et al., 2015), which may induce hyperactivity, weight loss, goiter, reduction in appetite, and interference with the reproductive process (MacLaurin et al., 1981; Wardatun et al., 2020). MacLaurin et al. (1981) reported that monogastric animals cannot neutralize the toxicity of mimosine. However, there are reports in the literature suggesting that the toxicity of mimosine can be addressed via several methods. Inoculation of cattle with mimosine-degrading rumen bacterium, Synergesties jonesii, can convert 3H4P and 2,3-DHP into harmless products and render cattle with the ability to avoid mimosine toxicity (Jones and Megarrity, 1986). Literature also suggests that soaking the harvested foliage for 24 h in water removes 99 % of mimosine (Soedarjo and Borthakur, 1996). Similarly, post-harvest storage of leucaena for up to 72 h at room temperature did not seem to affect the nutritional and structural contents of the foliage. However, it reduced mimosine content by 25%, which can be attributed to the mimosine-degrading enzyme, mimosinase, which remains active in the plant even after harvest (Bageel and Borthakur, 2022).
Applications
Leucaena is being used as a nutritious forage crop, agroforestry species for enhancing soil fertility in the degraded regions, and a source of numerous commercial and non-commercial ecosystem services in many sub-tropical, tropical, semi-arid, and Mediterranean regions of the world (Yousif et al., 2020). The provisioning services provided by leucaena include fodder, medicine, timber, firewood, high-quality paper, gum, green manure, fence posts, windbreaks, shade, biochar, biodiesel, and raw material for versatile industrial uses (Yusuff et al., 2019; Bageel et al., 2020; Yousif et al., 2020; De Angelis et al., 2021; Ibrahim et al., 2021). In terms of regulating services, leucaena can fix nitrogen and control soil erosion (Bageel et al., 2020). The cultural services provided by leucaena include landscaping and providing aesthetic beauty, particularly in urban environments (Sartori et al., 2019). Similarly, among the supporting services, leucaena is mainly known to regulate soil nutrient cycling and enhance soil fertility (Kumar et al., 1998; Isaac et al., 2003).
As a perennial legume, it is an important food source for feeding ruminants and chicken breeds due to its high palatability, optimal nutritional characteristics (high content of crude protein and non-structural carbohydrates), and fast growth rate (Verdecia et al., 2020; Thamaga et al., 2021). It fulfills the conditions to be a good nutraceutical (Marie-Magdeleine et al., 2020) and the inclusion of leucaena into a grass-based diet positively affects rumen fermentation, reduces CH4 formation, and enhances beneficial fatty acids (Irawan et al., 2020). In earlier times, leucaena was used to treat stomach diseases, facilitate abortion, contraception, and cure diabetes (Chowtivannakul et al., 2016). Current research also advocates its antidiabetic, anti-inflammatory, anthelmintic and antioxidant potential (Chowtivannakul et al., 2016; Zarin et al., 2016; Figueiredo et al., 2020). It has also been reported as a potent therapeutic agent for preventing and treating oral cancer (Chung et al., 2017).
It is an ideal plant species for the reforestation of marginal lands and watersheds as it can fix nitrogen, thrive on steep slopes, and control soil erosion (Bageel et al., 2020). Studies incorporating leucaena in agroforestry as an alley crop, mulch, or biochar showed higher crop yields, improved soil nutrient profile and suppression of weed growth (Heineman et al., 1997; Kumar et al., 1998; Isaac et al., 2003; Elias et al., 2020). Studies have also implicated the potency of leucaena for phytoremediation (Ávila et al., 2020; Bomfim et al., 2021) and wastewater treatment (Kristanda et al., 2021). It possesses inherent characteristics, e.g., the ability to survive in harsh environmental conditions and in a wide range of ecological settings, fast growth and reproductive rate, high phytomass production that can accumulate a large amount of heavy metals, and active coppicing that reduces the costs of replantation (Ssenku et al., 2017).
Invasive Attributes
Invasion Status
Leucaena is considered a conflict species owing to its strong invasive potential and useful agroforestry attributes. Although, of the three known sub-species of leucaena, only ssp. leucocephala is considered invasive; however, a large portion of literature does not differentiate between the two subspecies. Here we assume that most of the reports related to the invasion of leucaena address ssp. leucocephala. We also recommend the precise and absolute identification of the subspecies/cultivar of leucaena for future research purposes, as it will facilitate the betterment of risk assessment and course of action.
Leucaena has been widely introduced across the globe through various national and international forestry and forage programs (Brewbaker, 1987; Harris et al., 1994; González-García et al., 2009). The plant is enlisted in the top five terrestrial invasive plant species with the greatest international presence by Turbelin et al. (2017). Its presence has been recorded in the 28 of 30 Oceanic Islands surveyed by Kueffer et al. (2010). However, as far as its invasion status is concerned, it is not regarded as invasive in every country it has been introduced to, as some categorize it as “contentious” (Clarkson et al., 2010; Campbell et al., 2019). On the contrary, several other countries consider it an aggressive invasive species of the tropical and subtropical regions (Richardson and Rejmánek, 2011). As per CABI (2021), the plant is considered invasive in several parts of Africa (17 countries and Island nations), Asia (17), Europe (1), Oceania (23), North America (12), and South America (7) (Table 2). Based on its rate of spread and severe impacts on biodiversity, it has been recognized as one of the world's 100 worst invasive species (GISD, 2021).
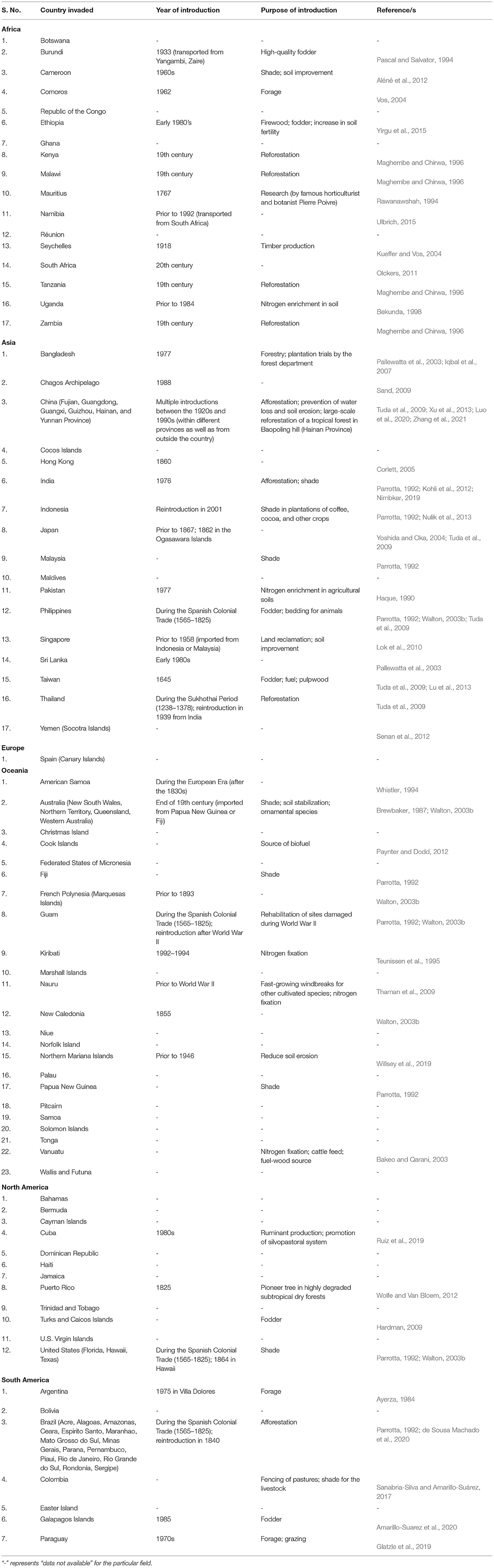
Table 2. List of countries in which Leucaena leucocephala is considered invasive (modified from CABI (2021)).
Most countries or Islands where the plant has emerged as invasive have introduced it deliberately for different reasons. For example, it was introduced multiple times in several regions of China between the 1920s and 1990s for afforestation, and to prevent water loss, and soil erosion (Tuda et al., 2009; Xu et al., 2013). On Hainan Island (China), it was used for the large-scale reforestation of a tropical forest in Baopoling hill, which was damaged due to extensive mining and industrial processes (Luo et al., 2020; Zhang et al., 2021). In India, leucaena was intentionally planted throughout the country for afforestation purposes (Kohli et al., 2012), and at present, its severe infestations can be observed in north-eastern regions and western Himalayas of India (Sekar, 2012; Debnath and Debnath, 2017). In Taiwan, the plant was introduced in 1,645 for household uses such as fodder, fuel, and pulp, and it widely proliferated in the lowlands of the country in the 1900s (Lu et al., 2013).
In South Africa, leucaena has been present since the 20th century. However, its spread is limited to low altitudinal regions with the highest densities being observed in the Durban-Tongaat area and scattered populations being reported from southwards in the Eastern Cape and northwards in Mozambique (Olckers, 2011). In Colombia, leucaena was planted in the pastures for fencing and providing shade to the livestock, and later, it spread throughout the tropical forest biome (Sanabria-Silva and Amarillo-Suárez, 2017). In Brazil, the introduction of leucaena was supported by the state of São Paulo in 1940 (de Sousa Machado et al., 2020) and now, the plant is recognized as a present and potentially invasive species of its different ecoregions, i.e., Atlantic forests, Caatinga, Cerrado, Amazon Mangroves, Southern Atlantic Mangroves, Atlantic Dry forests, Araucaria Moist forests, Dry Chaco, and Pantanal (Marques et al., 2020). The history of the introduction of leucaena in different countries where the plant is considered invasive is provided in Table 2.
Invasion Potential
Numerous functional attributes such as year-long flowering and fruiting, self-compatibility, massive propagule production, persistent seed bank, tolerance to the environmental stresses, ability to establish in the disturbed lands, and regeneration ability ensure the successful establishment, naturalization, and invasion of leucaena in the non-native habitats (Orwa et al., 2009; Marler, 2020). Further, the physiological and biochemical adaptations ensure its adaptability under environmental stresses (da Silva Rodrigues-Corrêa et al., 2019). Luo et al. (2020) observed that leucaena outperformed eight dominant native species in the tropical forests of Hainan Island (China) in terms of photosynthetic rate, transpiration rate, stomatal conductance, and osmotic/water stress tolerance. Further, leucaena is a tetraploid, and polyploid species usually have a higher survival and predisposed adaptability to the novel conditions of invaded habitats (Te Beest et al., 2012). High promiscuity of leucaena has also been reported by Ramírez-Bahena et al. (2020), which allows it to establish symbiosis with rhizobia of the different nativity as that of the plant, thereby increasing its invasive potential.
According to Chou and Kuo (1986), leucaena suppresses the growth of other woody plant species in its vicinity and herbaceous species in its understorey cover through allelopathy. Leaf litter of leucaena has been reported to reduce the seed germination and seedling growth in maize (Zea mays L.) under laboratory conditions (Singh et al., 1999). Likewise, the allelopathic effect of leucaena has also been reported on rice (Oryza sativa L.), soybean (Glycine max (L.) Merr.), radish (Raphanus sativus L.), cucumber (Cucumis sativus L.), and several weed species [e.g., Ageratum conyzoides L., Tridax procumbens L., Emilia sonchifolia (L.) DC. ex Wight, Eichhornia crassipes (Mart.) Solms, Medicago sativa L., and Cynodon dactylon (L.) Pers.] (Chaturvedi and Jha, 1992; Parvin et al., 2011; Chai et al., 2013; Kalpana and Navin, 2015; de Mattos Ribeiro et al., 2017; Chen et al., 2018). Phytotoxic allelochemicals present in the leachates of leucaena were reported to increase the electrolyte leakage and production of reactive oxygen species, decrease the membrane stability and cellular respiration, and modulate the activities of catalase and ascorbate peroxidase in the test plants (Chai et al., 2013).
Invasion of leucaena in the introduced regions is also determined by different environmental factors like temperature, precipitation, anthropogenic influence, and landscape characteristics (altitude and aspect, proximity to river/road/agricultural land/forest edges, and forest type) (Chiou et al., 2013). Of these factors, temperature, precipitation, and altitude play the most influential role in the spread of leucaena (Chiou et al., 2013). Most often, leucaena has been found to dominate the areas near riverbanks, roadsides, agricultural lands, forest edges, and wastelands (Chiou et al., 2013). In South Africa, the maximum invasion of the plant has been witnessed in Savanna Biome, followed by watercourses and wetlands, forest habitats, and Grassland Biome (Olckers, 2011). Natural and anthropogenic disturbances also have an important role in enhancing the invasion potential of leucaena. The average annual rate of spread of leucaena was 3.55 ha in disturbed and abandoned areas (Chen et al., 2012). Rapid changes in the land use pattern have elevated the impact of leucaena in the Hengchun peninsula in Taiwan (Chiou et al., 2016). With increasing shifts in seasonal and land-use patterns, the invasion potential of leucaena is likely to be aggravated in the near future. According to the climatic suitability study assessing the projected spread of 41 invasive species in Sri Lanka, leucaena showed the maximum potential of spread after Panicum maxicum Jacq. and Lantana camara L. (Kariyawasam et al., 2020). A high probability of its distribution has also been predicted throughout Latin America, central and southern Africa, southeastern Asia, eastern Australia, New Zealand, and Western Europe, particularly in the protected areas (Wan et al., 2018).
Impact of Invasion
Leucaena suppresses the growth of resident woody species and understory vegetation, thereby reducing species diversity and richness. It has been reported to form thickets or monospecific stands that threaten the rare and endemic plant species (Costa et al., 2015; Campbell et al., 2019). A field study by Jurado et al. (1998) has proven the harmful effect of leucaena plantation on the seedling establishment of resident species. Leucaena inhibits light transmittance to the forest floor due to excessively broad and bushy growth, which hampers natural forest regeneration (Marod et al., 2012). Yoshida and Oka (2004) reported that invasion by leucaena has altered the second successional pathway and reduced the biodiversity in late-successional forests of the Ogasawara (Bonin) Islands. Similarly, Hata et al. (2007) also reported reduced seed germination and seedling growth of Schima mertensiana Koidz. in sites dominated by leucaena, compared to those dominated by Trema orientalis (L.) Blume, indicating a potential shift in the pattern of early succession in the region. The plant has been known to modify the forest and savannah ecosystems of Brazil in different manners, producing an irreversible impact (Mignoni et al., 2018).
Monospecific stands of leucaena in the invaded forest ecosystems also increased the mineralization of elements in the soil and decreased its ability to sequester recalcitrant carbon and nitrogen pools (Marler et al., 2016; Marler, 2020). The soil properties of the Mariana Islands of Guam were changed by long-term infestations of leucaena and other invasive species to the extent that long-term mitigation and restoration activities are required for the below-ground ecosystems to return to their native state (Marler, 2020). Invasion by leucaena has driven the abandonment of farmlands and uprising of industrialization in Penghu Island, Taiwan, and such land-use changes have influenced both the native flora and fauna and increased the vector-borne diseases (Wei et al., 2020).
Management
So far, only the conventional techniques have been used to manage leucaena, which include mechanical, chemical, and biological control methods. Mechanical methods include cutting the adult trees followed by shading/covering the trunk with plastic sheets or trunk girdling to reduce the emergence of new sprouts (Peng et al., 2019). If newly emerging sprouts are consistently managed, and the targeted area is rehabilitated by suitable native vegetation, the method can effectively manage the spread and regeneration of leucaena. However, the scheme is usually expensive, labor-intensive, and requires consistent efforts for longer durations. It has also been observed that bulldozing and similar anthropogenic disturbances facilitate the quick regeneration and seedling recruitment of the species (Jaime et al., 2017). This will demand additional and repetitive efforts for its complete removal. In addition, natural management of the plant via regular grazing is another viable option, but leucaena has displayed a remarkable resilience toward frequent grazing, which otherwise can only control the vegetative growth of the plant (Idol, 2019).
Chemical control includes methods like repeated herbicide application to cut the surface of stumps or basal barks, herbicide smearing on the girdled area of the trunk, and injecting herbicide into the trunk every season (Peng et al., 2019). However, the selection of chemicals should be done carefully as the plant is resistant to most commonly used herbicides (Idol, 2019). Studies have reported its sensitivity toward glyphosate (Peng et al., 2019), triclopyr, picloram (Idol, 2019), and metsulfuron-methyl (Olckers, 2011). However, the efficacy of herbicides reduces during the rainy season (Peng et al., 2019) and the chemical treatments are often a subject of conflict, due to their environmental implications.
Under the biological control methods, interactions of leucaena with its natural enemies, Heteropsylla cubana Crawford (leaf-sucking psyllid) and Acanthoscelides macrophthalmus Schaeffer (seed-boring bruchid beetle) have been studied extensively in Australia (Raghu et al., 2005) and South Africa (Olckers, 2011). However, studies have revealed that H. cubana is inefficient in managing infestations of the plant (Olckers, 2011). Further, A. macrophthalmus alone was also not found to be sufficient on its own in regulating the populations of leucaena as it causes only modest levels of seed damage (Olckers, 2011; Sharratt and Olckers, 2012). In addition, these biocontrol agents coexist naturally with leucaena in several invaded regions and are unable to manage its spread and impact (Idol, 2019). This indicates that it is not sufficient to rely on these pests to manage leucaena. Additional pests and pathogens, mainly the insects that exploit green pods, are required to manage infestations of the plant (Sharratt and Olckers, 2019). Certain native plant species may also inflict a phytotoxic effect on leucaena. For instance, seed leachates of a tropical native legume, Sesbania virgata (Cav.) Pers. from Brazil, Argentina, Uruguay, and Paraguay have been reported to suppress germination and seedling growth in leucaena (Mignoni et al., 2018). Interestingly, the allelopathic effect of leucaena on S. virgata was almost negligible (Mignoni et al., 2018). Similarly, a recent study using a trait-based framework and software selected Bougainvillea spectabilis Willd., a native plant species of China, for the biocontrol of leucaena (Zhang et al., 2021). The efficacy of the selection process was tested by planting its seedlings in the vicinity of leucaena for 3 years, which significantly restricted the spread of leucaena (Zhang et al., 2021). To replace the species, it is important to identify such native species with a similar growth pattern and drought stress tolerance traits as that of leucaena (Luo et al., 2020).
An integrated approach constituting multiple conventional techniques may prove effective in controlling the spread of leucaena. An appropriate combination of control methods, correct timing of application, and selection of a suitable target area are important. Hwang et al. (2010) suggested that the summer season is ideal to control the species as seedling recruitments occur during this period. Since the likelihood of invasion is higher in low-elevation areas with proximity to roads, water, and forest edges, these habitats should be monitored and targeted on a priority basis (Chiou et al., 2013). Recently, Osawa et al. (2019) stated that adequate propagules supply and habitat suitability are the most critical aspects for successful invasion in an area. Therefore, a framework was proposed for devising management strategies based on propagules supply and suitable habitat for leucaena, where the target areas for management are classified under four classes. Class I represented the adequate propagule supply and adequate habitat suitability; Class II represented the adequate propagule supply and limited habitat suitability; Class III represented the limited propagule supply and adequate habitat suitability; and Class IV represented the limited propagule supply and limited habitat suitability. The authors suggested that classifying the target areas would facilitate prioritization and implementation of management practices (Osawa et al., 2019). Predictions about species distribution using climate suitability models can also help conservation managers to develop proactive management plans (Chiou et al., 2016). Efforts toward educating the people who are directly or indirectly involved in the cultivation or management of leucaena will provide a strong uplift to the management and restoration practices (Chiou et al., 2013).
At the same time, it is obligatory to refute the rampant use of ssp. leucocephala without considering its invasive propensities so that problems that would arise from subsequent invasions can be avoided. Alternatives should be explored to replace the species to continue the flow of ecosystem services provided by the tree while minimizing the environmental risks. The other subspecies of leucaena, i.e., ssp. glabrata is also an important nitrogen-fixing tree legume used for agroforestry and animal husbandry (Bageel et al., 2020). Although ssp. glabrata is a large tree that can grow up to 20 m in height, but it can be maintained as a dwarf bush via repetitive harvesting for fodder purposes (Honda et al., 2018). Ecological adaptability of ssp. glabrata is also comparable with ssp. leucocephala (Honda et al., 2018). Therefore, replacing ssp. leucocephala with ssp. glabrata may provide better options for animal feed production, agroforestry, and the development of other industrial products without being a threat to the native plant diversity. Though ssp. glabrata has not been reported invasive up till now, a few studies suggest that it has the potential to become invasive in the future (Olckers, 2011; Campbell et al., 2019). It is important to ensure that ssp. glabrata will not emerge as a troublesome invader before using it as an alternative for ssp. leucocephala. To be safer, other native or non-invasive naturalized species that could provide similar benefits should be selected and promoted over leucaena. The selection of such species should be driven by geographical status and ethnobotanical importance of a species, local datasets and informants, and long-term observations. Moreover, with the help of extensive genetic analyses and novel molecular techniques, the next generation of multipurpose leucaena cultivars, especially the sterile ones, is currently underway, which may amplify its commercial usage and nullify the environmental impacts (Dalzell, 2019).
Conclusions and Way Forward
Leucaena is a commercially important agroforestry species, which has been introduced worldwide for its provisional services, restoration of degraded and contaminated lands, and prevention of water loss and soil erosion (Luo et al., 2020). However, soon after its introduction, ssp. leucocephala turned invasive in many regions and threatened the persistence of resident flora and fauna. Although there are many reports validating the invasion potential of the plant and the threats it has been posing to the biodiversity, its propagation for agroforestry, forage and restoration purposes are still being advocated without proper caution (Wolfe and Van Bloem, 2012; Edwin-Wosu and Nkang, 2016).
The lack of awareness and/or ignorance toward the long-lasting ecological problems that might follow the unprecedented propagation of ssp. leucocephala is a much larger issue that needs to be resolved on a priority basis. The enormity of economic costs and human resources associated with the eradication programs for invasive species are usually higher than the socio-economic benefits associated with them. Accompanied with these are the ecological impacts, some of which might be irreversible. Therefore, further propagation of ssp. leucocephala outside its indigenous geographic range should be reconsidered. Depending upon posed risk of travel, both legislative and non-legislative measures can be adopted to keep its population in check (Campbell et al., 2019). In the regions where it is impossible to prohibit further plantations or where invasive behavior of the plant has not been reported yet, it is important to devise suitable monitoring systems to inspect its spread and impacts. Further, propagation of non-invasive subspecies and hybridized cultivars of leucaena should be done carefully. There might be a strong possibility that the plant may have not realized its full invasive potential because of suboptimal environmental conditions or extensive browsing by animals. The invasive plant species usually undergo a lag phase before their populations can rise rapidly (Wolfe and Van Bloem, 2012). With the seasonal shifts and climate warming, the infestations of leucaena may augment in near future and the plant may expand its invasion range in presently uninvaded territories.
Moreover, it is important to understand that most of the benefits associated with the plantations of exotic species are potential or perceived rather than real (Jurado et al., 1998). These benefits can be easily obtained from native species; only the selection needs to be done wisely. Sometimes, the difference between studies reporting beneficial aspects of an exotic plant and those reporting its impacts is usually vast with more publications siding with the beneficial aspects. This may often result in knowledge gaps, partial insights into the facts, and biased decisions. Therefore, the research in both directions should be encouraged and supplemented, and the impacts of invasive exotic species should be extensively explored along with their beneficial aspects. Bridging these knowledge gaps will ensure a better evaluation of pros and cons, strengthening of individual perspectives, an efficient decision-making process, and avoidance of future conflicts.
Author Contributions
PS wrote the original draft. AK, DB, SK, and BC edited the paper. DB and BC conceptualized the review. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are thankful to National Mission on Himalayan Studies (NMHS), Ministry of Environment, Forest and Climate Change (MoEF&CC), New Delhi, India, and the University of Queensland, Queensland, Australia for financial assistance.
References
Agriculture Victoria. (2021). Victorian Resources Online, Leucaena leucocephala. Available online at: http://vro.agriculture.vic.gov.au/dpi/vro/vrosite.nsf/pages/weeds_leucaena (accessed May 28, 2021).
Aléné, D. C, Djiéto-Lordon, C., Tadu, Z., and Burckhardt, D. (2012). Infestation of Heteropsylla cubana (Hemiptera: Psylloidea) on Leucaena leucocephala (Fabaceae: Mimosoideae) in Cameroon. Afr. Entomol. 20, 207–216. doi: 10.4001/003.020.0204
Amarillo-Suarez, A. R., Camacho-Erazo, M., Morse, G., Rueda, D., and Herrera, H. W. (2020). New distribution records and host plant associations of Bruchinae (Coleoptera: Chrysomelidae) in the Galapagos Islands, with a revised checklist of species and their associated host plants. Coleopt. Bull. 74, 719–723. doi: 10.1649/0010-065X-74.4.719
Ávila, P. A., Faquin, V, Ávila, F. W., Kachinski, WD, Carvalho, G. S., and Guilherme, L. R. G. (2020). Phosphorus and sulfur in a tropical soil and their effects on growth and selenium accumulation in Leucaena leucocephala (Lam.) de Wit. Environ. Sci. Pollut. Res. 27, 44060–44072. doi: 10.1007/s11356-020-10303-3
Awang, A. N., Mohamed, A. R., Salleh, N. H. M., Hoo, P. Y., and Kasim, N. N. (2019). Torrefaction of Leucaena leucocephala under isothermal conditions using the Coats–Redfern method: kinetics and surface morphological analysis. React. Kinet. Mech. Catal. 128, 663–680. doi: 10.1007/s11144-019-01659-w
Ayerza, R. (1984). Introduction of Leucaena leucocephala in the arid chaco of villa Dolores, Argentina. Leucaena Res. Rep. 5, 1.
Bageel, A., Honda, M. D. H., Carrillo, J. T., and Borthakur, D. (2020). Giant leucaena (Leucaena leucocephala subsp. glabrata): a versatile tree-legume for sustainable agroforestry. Agrofor. Syst. 94, 251–268. doi: 10.1007/s10457-019-00392-6
Bageel, A. M., and Borthakur, D. (2022). The effects of pH, salinity, age of leaves, post-harvest storage duration, and psyllid infestation on nutritional qualities of giant leucaena fodder. J. Crop Sci. Biotechnol. doi: 10.1007/s12892-021-00139-9
Bakeo, R., and Qarani, F. (2003). “Country report on the forestry invasive species situation in Vanuatu,” in Proceedings of the Asia-Pacific Forest invasive Species Conference, The unwelcome guests, Country Reports. Yunnan Province, China: Kunming. Available online at: http://www.fao.org/3/ae944e/ae944e0a.htm (accessed May 28, 2021).
Batisteli, A. F., Costa, R. O., and Christianini, A. V. (2020). Seed abundance affects seed removal of an alien and a native tree in the Brazilian savanna: implications for biotic resistance. Austral Ecol. 45, 1007–1015. doi: 10.1111/aec.12922
Bekunda, M. (1998). Nitrogen fixation in Leucaena leucocephala and effects of prunings on cereal yields. Technical report IAEA-TECDOC-1053. Vienna, Austria: Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture, p. 33–40.
Bomfim, N. C. P., Aguilar, J. V., de Paiva, W. D. S., de Souza, L. A., Justino, G. C., Faria, G. A., et al. (2021). Iron phytostabilization by Leucaena leucocephala. S. Afr. J. Bot. 138, 318–327. doi: 10.1016/j.sajb.2021.01.013
Brewbaker, J. L. (1987). “Leucaena: a multipurpose tree genus for tropical agroforestry”, in Agroforestry: A Decade of Development, Steppler, H. A, and Nair, P. K. R., eds. Nairobi, Kenya: International Council for Research in Agroforestry. p. 289–323.
Buck, S., Rolfe, J., Lemin, C., and English, B. (2019). Adoption, profitability and future of leucaena feeding systems in Australia. Trop. Grassl. 7, 303–314. doi: 10.17138/TGFT(7)303-314
CABI. (2021). Invasive Species Compendium: Leucaena leucocephala. Available online at: https://www.cabi.org/isc/datasheet/31634 (accessed May 28, 2021)
Campbell, S., Vogler, W., Brazier, D., Vitelli, J., and Brooks, S. (2019). Weed leucaena and its significance, implications and control. Trop. Grassl. 7, 280–289. doi: 10.17138/TGFT(7)280-289
Chai, T. T., Ooh, K. F., Ooi, P. W., Chue, P. S., and Wong, F. C. (2013). Leucaena leucocephala leachate compromised membrane integrity, respiration and antioxidative defence of water hyacinth leaf tissues. Bot. Stud. 54, 8. doi: 10.1186/1999-3110-54-8
Chaturvedi, O. P., and Jha, A. N. (1992). Studies on allelopathic potential of an important agroforestry species. For. Ecol. Manag. 53, 91–98. doi: 10.1016/0378-1127(92)90036-9
Chen, F., Liu, K., Xie, Z., Liu, M., and Chen, C. (2018). Effects of decomposing leaf litter of Leucaena leucocephala on photosynthetic traits of Cynodon dactylon and Medicago sativa. New For. 49, 667–679. doi: 10.1007/s11056-018-9651-7
Chen, J. C., Chen, C. T., and Jump, A. S. (2012). Forest disturbance leads to the rapid spread of the invasive Leucaena leucocephala in Taiwan. Int. Arch. Photogramm. Remote. 39, 35–40. doi: 10.5194/isprsarchives-XXXIX-B2-35-2012
Chiou, C. R., Chen, Y. J., Wang, H. H., and Grant, W. E. (2016). Predicted range expansion of the invasive plant Leucaena leucocephala in the Hengchun peninsula, Taiwan. Biol. Invas. 18, 381–394. doi: 10.1007/s10530-015-1010-4
Chiou, C. R., Wang, H. H., Chen, Y. J., Grant, W. E., and Lu, M. L. (2013). Modeling potential range expansion of the invasive shrub Leucaena leucocephala in the Hengchun peninsula, Taiwan. Invasive Plant Sci. Manag. 6, 492–501. doi: 10.1614/IPSM-D-13-00010.1
Chou, C. H., and Kuo, Y. L. (1986). Allelopathic research of subtropical vegetation in Taiwan. III. Allelopathic exclusion of understorey by Leucaena leucocephala (Lam.) de Wit. J. Chem. Ecol. 12, 1431–1448. doi: 10.1007/BF01012362
Chowtivannakul, P., Srichaikul, B., and Talubmook, C. (2016). Antidiabetic and antioxidant activities of seed extract from Leucaena leucocephala (Lam.) de Wit. Agric. Nat. Resour. 50, 357–361. doi: 10.1016/j.anres.2016.06.007
Chung, H. H., Chen, M. K., Chang, Y. C., Yang, S. F., Lin, C. C., and Lin, C. W. (2017). Inhibitory effects of Leucaena leucocephala on the metastasis and invasion of human oral cancer cells. Environm. Toxicol. 32, 1765–1774. doi: 10.1002/tox.22399
Clarkson, J. R., Grice, A. C., Friedel, M. H., Setterfield, S. A., and Ferdinands, K. (2010). “The role of legislation and policy in dealing with contentious plants,” in Proceedings of the 17th Australasian Weeds Conference. p. 474–477. Available online at: https://core.ac.uk/download/pdf/47198728.pdf (accessed May 28, 2021).
Corlett, R. T. (2005). Interactions between birds, fruit bats and exotic plants in urban Hong Kong, South China. Urban Ecosyst. 8, 275–283. doi: 10.1007/s11252-005-3260-x
Costa, J. T., Fonseca, I. C. B., and Bianchini, E. (2015). Population structure of the invasive species Leucaena leucocephala (Fabaceae) in a seasonal semi-deciduous forest, southern Brazil. Aust. J. Botany. 63, 590–596. doi: 10.1071/BT14308
da Silva Rodrigues-Corrêa, K. C., Honda, M. D., Borthakur, D., and Fett-Neto, A. G. (2019). Mimosine accumulation in Leucaena leucocephala in response to stress signaling molecules and acute UV exposure. Plant Physiol. Biochemist. 135, 432–440. doi: 10.1016/j.plaphy.2018.11.018
Dalzell, S. A. (2019). Leucaena cultivars-current releases and future opportunities. Trop. Grassl. 7, 56–64. doi: 10.17138/TGFT(7)56-64
De Angelis, A., Gasco, L., Parisi, G., and Danieli, P. P. (2021). A multipurpose leguminous plant for the Mediterranean countries: Leucaena leucocephala as an alternative protein source: A review. Animals. 11, 2230. doi: 10.3390/ani11082230
de Mattos Ribeiro, V., Spiassi, A., Marcon, T. R., de Lima, G. P., Corsato, J. M., and Fortes, A. M. T. (2017). Antioxidative enzymes of Cucumis sativus seeds are modulated by Leucaena leucocephala extracts. Acta Scientiarum. Biol. Sci. 39, 373–380. doi: 10.4025/actascibiolsci.v39i3.34801
de Sousa Machado, M. T., Drummond, J. A., and Barreto, C. G. (2020). Leucaena leucocephala (Lam.) de Wit in Brazil: history of an invasive plant. Estudos Ibero-Americanos 46, 1–20. doi: 10.15448/1980-864X.2020.1.33976
Debnath, A., and Debnath, B. (2017). Diversity, invasion status and usages of alien plant species in northeastern hilly state of Tripura: a confluence of Indo-Barman hotspot. Am. J. Plant Sci. 8, 212–235. doi: 10.4236/ajps.2017.82017
Edwin-Wosu, N. L., and Nkang, A. (2016). Evaluation of phytoremediation potential of Peltophorum pterocarpum (DC.) Heyne Leucaena leucocephala (Lam.) De Wit. and Crotolaria retusa Linn for waste oil contaminated soils. J. Appl. SCI. Environ. 20, 669–678. doi: 10.4314/jasem.v20i3.22
Elias, D. M. O., Ooi, G. T., Razi, M. F. A., Robinson, S., Whitaker, J., and McNamara, N. P. (2020). Effects of Leucaena biochar addition on crop productivity in degraded tropical soils. Biomass Bioenergy. 142, 105710. doi: 10.1016/j.biombioe.2020.105710
Figueiredo, B. N. S., Sato, M. O., Moura, L. T. S., Mariano, S. M. B., Alvim, T. C., Soares, I. M., et al. (2020). Preliminary report on the effect of Savanna plants Leucaena leucocephala, Parkia platycephala and Senna alata against eggs and immature stages of trichostrongylid nematodes in vitro. Pathogens. 9, 986. doi: 10.3390/pathogens9120986
Gaskin, J. F. (2017). The role of hybridization in facilitating tree invasion. AoB Plants. 9, plw079. doi: 10.1093/aobpla/plw079
GISD. (2021). Global Invasive Species Database, 100 of the world's worst invasive alien species. Available online at: http://www.iucngisd.org/gisd/100_worst.php (accessed May 28, 2021).
Glatzle, A. F., Cabrera, A. N., Naegele, A., and Klassen, N. (2019). Leucaena feeding systems in Paraguay. Trop. Grassl. 7, 397–402. doi: 10.17138/TGFT(7)397-402
González-García, E., Cáceres, O., Archimède, H., and Santana, H. (2009). Nutritive value of edible forage from two Leucaena leucocephala cultivars with different growth habit and morphology. Agrofor. Syst. 77, 131–141. doi: 10.1007/s10457-008-9188-4
Haque, S. M. S. (1990). Nitrogen fixing trees and shrubs of Pakistan and their role in soil improvement—a review. Commonw. For. Rev. 69, 345–349.
Hardman, C. (2009). Invasive plants in the Turks and Caicos Islands. Masters' Dissertation, Imperial College of London. Available online at: https://www.iccs.org.uk/wp-content/thesis/consci/2009/Hardman.pdf (accessed May 28, 2021).
Harris, S. A., Hughes, C. E., Abbott, R. J., and Ingram, R. (1994). Genetic variation in Leucaena leucocephala (Lam.) de Wit. (Leguminosae: Mimosoidae). Silvae Genetica. 43, 159–167.
Hata, K., Suzuki, J. I., and Kachi, N. (2007). Effects of an alien shrub species, Leucaena leucocephala, on establishment of native mid-successional tree species after disturbance in the national park in the Chichijima Island, a subtropical oceanic island. Tropics. 16, 283–290. doi: 10.3759/tropics.16.283
Heineman, A. M., Otieno, H. J. O., Mengich, E. K., and Amadalo, B. A. (1997). Growth and yield of eight agroforestry tree species in line plantings in Western Kenya and their effect on maize yields and soil properties. For. Ecol. Manag. 91, 103–135. doi: 10.1016/S0378-1127(96)03885-6
Honda, M. D., and Borthakur, D. (2019). Mimosine concentration in Leucaena leucocephala under various environmental conditions. Trop. Grassl. 7, 164–172. doi: 10.17138/TGFT(7)164-172
Honda, M. D., and Borthakur, D. (2020). Mimosine facilitates metallic cation uptake by plants through formation of mimosine–cation complexes. Plant Mol. Biol. 102, 431–445. doi: 10.1007/s11103-019-00956-1
Honda, M. D., and Borthakur, D. (2021). Mimosine is a stress-response molecule that serves as both an antioxidant and osmolyte in giant leucaena (Leucaena leucocephala subsp. glabrata) during environmental stress conditions. Plant Stress. 2, 100015. doi: 10.1016/j.stress.2021.100015
Honda, M. D. H., Ishihara, K. L., Pham, D. T., and Borthakur, D. (2018). Identification of drought-induced genes in giant leucaena (Leucaena leucocephala subsp. glabrata). Trees. 32, 571–585. doi: 10.1007/s00468-018-1657-4
Hwang, C. Y., Hsu, L. M., Liou, Y. J., and Wang, C. Y. (2010). Distribution, growth, and seed germination ability of lead tree (Leucaena leucocephala) plants in Penghu Islands, Taiwan. Weed Technol. 24, 574–582. doi: 10.1614/WT-D-10-00042.1
Ibrahim, W. M. H. W., Amini, M. H. M., Sulaiman, N. S., and Kadir, W. R. W. A. (2021). Evaluation of alkaline-based activated carbon from Leucaena leucocephala produced at different activation temperatures for cadmium adsorption. Applied Water Sci. 11, 1. doi: 10.1007/s13201-020-01330-z
ICAR. (2006). Leucaena leucocephala (Subabool) A Promising Forage Plant. Available online at: https://tripuraicar.nic.in/publication/AGRICULTURE%2002/A%20PROMISING%20FORAGE%20PLANT.pdf (accessed May 28, 2021).
Idol, T. (2019). A short review of leucaena as an invasive species in Hawaii. Trop. Grassl. 7, 290–294. doi: 10.17138/TGFT(7)290-294
Ilham, Z., Hamidon, H., Rosji, N. A., Ramli, N., and Osman, N. (2015). Extraction and quantification of toxic compound mimosine from Leucaena leucocephala leaves. Procedia Chemi. 16, 164–170. doi: 10.1016/j.proche.2015.12.029
Iqbal, G. M. A., Huda, S. M. S., Sujauddin, M., and Hossain, M. K. (2007). Effects of sludge on germination and initial growth performance of Leucaena leucocephala seedlings in the nursery. J. For. Res. 18, 226–230. doi: 10.1007/s11676-007-0046-4
Irawan, A., Noviandi, C. T., Kustantinah, W.idyobroto B. P., Astuti, A., and Ates, S. (2020). Effect of Leucaena leucocephala and corn oil on ruminal fermentation, methane production and fatty acid profile: an in vitro study. Anim. Prod. Sci. 61, 459–469. doi: 10.1071/AN20003
Isaac, L., Wood, C. W., and Shannon, D. A. (2003). Pruning management effects on soil carbon and nitrogen in contour-hedgerow cropping with Leucaena leucocephala (Lam.) De Wit on sloping land in Haiti. Nutr. Cycling Agroecosyst. 65, 256–263. doi: 10.1023/A:1022600720226
Jaime, X. A., Van Bloem, S. J., Koch, F. H., and Nelson, S. A. (2017). Spread of common native and invasive grasses and ruderal trees following anthropogenic disturbances in a tropical dry forest. Ecol. Process. 6, 38. doi: 10.1186/s13717-017-0103-7
Jones, R. J., and Megarrity, R. G. (1986). Successful transfer of DHP-degrading bacteria from Hawaiian goats to Australian ruminants to overcome the toxicity of Leucaena. Aust. Vet. J. 63, 259–262. doi: 10.1111/j.1751-0813.1986.tb02990.x
Jurado, E., Flores, J., Navar, J., and Jiménez, J. (1998). Seedling establishment under native tamaulipan thornscrub and Leucaena leucocephala plantation. For. Ecol. Manag. 105, 151–157. doi: 10.1016/S0378-1127(97)00276-4
Kalpana, P., and Navin, M. K. (2015). Assessment of allelopathic potential of Leucaena leucocephala (Lam) De Vit on Raphanus sativus L. Int. J. Sci. Res. Publ. 5, 1–3.
Kariyawasam, C. S., Kumar, L., and Ratnayake, S. S. (2020). Potential risks of plant invasions in protected areas of Sri Lanka under climate change with special reference to threatened vertebrates. Climate. 8, 51. doi: 10.3390/cli8040051
Kodiango, R. O., and Palapala, V. A. (2016). Effect of eco-physiological variability in Leucaena leucocephala growth and development. Int. J. Plant Soil Sci. 11, 1–10.
Kohli, R. K., Batish, D. R., Singh, J. S., Singh, H. P., and Bhatt, J. R. (2012). “Plant invasion in India: An overview”, in Invasive Alien Plants: An Ecological Appraisal for the Indian Subcontinent, Bhatt, J. R., Singh, J. S., Singh, S. P., Tripathi, R. S., and Kohli, R. K., (eds.). Wallingford, UK: CAB International. p. 1–9.
Kristanda, J., Sintiago, K. S., Kristianto, H., Prasetyo, S., and Sugih, A. K. (2021). Optimization Study of Leucaena leucocephala seed extract as natural coagulant on decolorization of aqueous Congo Red solutions. Arab J. Sci. Eng. 46, 6275–6286. doi: 10.1007/s13369-020-05008-1
Kueffer, C., Daehler, C. C., Torres-Santana, C. W., Lavergne, C., Meyer, J. Y., Otto, R., et al. (2010). A global comparison of plant invasions on oceanic islands. Perspect. Plant Ecol. Sys. 12, 145–161. doi: 10.1016/j.ppees.2009.06.002
Kueffer, C., and Vos, P. (2004). “Case studies on the status of invasive woody plant species in the western Indian Ocean: 5. Seychelles”, in Forest Health & Biosecurity Working Papers FBS/4-5E. Rome, Italy: Forestry Department, Food and Agriculture Organization of the United Nations. Available online at: http://www.fao.org/forestry/6848-07d9a941381eb5e556c4ead52e20bd418.pdf (accessed May 28, 2021)
Kumar, B. M., Kumar, S. S., and Fisher, R. F. (1998). Intercropping teak with Leucaena increases tree growth and modifies soil characteristics. Agrofor. Syst. 42, 81–89. doi: 10.1023/A:1006199910985
Lin, Y., Chen, A., Yan, S., Rafay, L., Du, K., Wang, D., et al. (2019). Available soil nutrients and water content affect leaf nutrient concentrations and stoichiometry at different ages of Leucaena leucocephala forests in dry-hot valley. J. Soils Sedim. 19, 511–521. doi: 10.1007/s11368-018-2029-9
Lok, A. F. S. L., Tan, K. X., Ang, W. F., and Tan, H. T. W. (2010). The distribution and ecology of Leucaena leucocephala (Lam.) de Wit ssp. leucocephala (Fabaceae) in Singapore. Cosmos. 6, 45–55. doi: 10.1142/S0219607710000462
Lu, M. L., Huang, J. Y., Chung, Y. L., and Huang, C. Y. (2013). Modelling the invasion of a Central American Mimosoid tree species (Leucaena leucocephala) in a tropical coastal region of Taiwan. Remote Sensing Lett. 4, 485–493. doi: 10.1080/2150704X.2012.755274
Luo, J., Cui, J., Pandey, S. P., Jiang, K., Tan, Z., He, Q., et al. (2020). Seasonally distinctive growth and drought stress functional traits enable Leucaena Leucocephala to successfully invade a Chinese tropical forest. Trop. Conservat. Sci. 13, 1–7. doi: 10.1177/1940082920949176
MacLaurin, A. R., Tainton, N. M., and Bransby, D. I. (1981). Leucaena leucocephala (lam.) de wit as a forage plant-a review. Proceed. Ann. Congr. Grassland Society S. Afr. 16, 63–69. doi: 10.1080/00725560.1981.9648922
Maghembe, J. A., and Chirwa, P. W. (1996). Growth and biomass production of some Leucaena leucocephala accessions grown at Makoka, Malawi. J. Trop. For. Sci. 8, 481–490.
Marie-Magdeleine, C., Ceriac, S., Barde, D. J., Minatchy, N., Periacarpin, F., Pommier, F., et al. (2020). Evaluation of nutraceutical properties of Leucaena leucocephala leaf pellets fed to goat kids infected with Haemonchus contortus. BMC Vet. Res. 16, 280. doi: 10.1186/s12917-020-02471-8
Marler, T. E. (2020). Three invasive tree species change soil chemistry in Guam forests. Forests 11, 279. doi: 10.3390/f11030279
Marler, T. E., Dongol, N., and Cruz, G. N. (2016). Leucaena leucocephala and adjacent native limestone forest habitats contrast in soil properties on Tinian Island. Communicative & Integrative Biol. 9:e1212792. doi: 10.1080/19420889.2016.1212792
Marod, D., Duengkae, P., Kutintara, U., Sungkaew, S., Wachrinrat, C., Asanok, L., et al. (2012). The influences of an invasive plant species (Leucaena leucocephala) on tree regeneration in Khao Phuluang Forest, northeastern Thailand. Kasetsart J. (Natural Science). 46, 39–50.
Marques, A. R., Costa, C. F., Atman, A. P. F., and Garcia, Q. S. (2014). Germination characteristics and seedbank of the alien species Leucaena leucocephala (Fabaceae) in Brazilian forest: ecological implications. Weed Res. 54, 576–583. doi: 10.1111/wre.12107
Marques, A. R., Lima, L. L., Garcia, Q. S., and Atman, A. P. F. (2020). A novel cellular automata approach: seed input/output of the alien species Leucaena leucocephala in the soil and the effects of climate changes. Plant Ecol. 221, 141–154. doi: 10.1007/s11258-019-00999-w
McMillan, H. E., Liu, G., Shelton, H. M., Dalzell, S. A., Godwin, I. D., Gamage, H., et al. (2019). Sterile leucaena becomes a reality? Trop. Grassl. 7, 74–79. doi: 10.17138/TGFT(7)74-79
Mignoni, D. S. B., Simões, K., and Braga, M. R. (2018). Potential allelopathic effects of the tropical legume Sesbania virgata on the alien Leucaena leucocephala related to seed carbohydrate metabolism. Biol. Invas. 20, 165–180. doi: 10.1007/s10530-017-1524-z
Ngongolo, K., Mtoka, S., and Mahulu, A. (2014). The Abundance and pollinators' impact on seed setting of Leucaena leucocephala in Wazo Hill restored Quarry, Tanzania. J. Zool. Biosci. 1, 6–10.
Nimbkar, N. (2019). Leucaena feeding systems in India. Trop. Grassl. 7, 415–419. doi: 10.17138/TGFT(7)415-419
Nulik, J., Hau, D. K., Edison, R. G., Pakaereng, C., and Liubana, D. (2013). “Farmer based seed production of Leucaena leucocephala in Eastern Indonesia”, in Proceedings of 22nd International Grassland Congress, Michalk, D. L., Millar, G. D., Badgery, W. B., and Broadfoot, K. M., (eds.). Orange New South Wales, Australia: New South Wales Department of Primary Industry. p. 444–445.
Olckers, T. (2011). Biological control of Leucaena leucocephala (Lam.) de Wit (Fabaceae) in South Africa: a tale of opportunism, seed feeders and unanswered questions. Afr. Entomol. 19, 356–365. doi: 10.4001/003.019.0219
Orwa, C., Mutua, A., Kindt, R., Jamnadass, R., and Simmons, A. (2009). Agroforestree Database: A Tree Reference and Selection Guide Version 4.0. Kenya: World Agroforestry Centre.
Osawa, T., Akasaka, M., and Kachi, N. (2019). Facilitation of management plan development via spatial classification of areas invaded by alien invasive plant. Biol. Invas. 21, 2067–2080. doi: 10.1007/s10530-019-01958-2
Pallewatta, N., Reaser, J. K., and Gutierrez, A. T. (2003). Invasive Alien Species in South-Southeast Asia: National Reports & Directory of Resources. Cape Town, South Africa: Global Invasive Species Programme. Available online at: https://www.doi.gov/sites/doi.gov/files/uploads/invasive_alien_species_in_south_southeast_asia.pdf (accessed on 28 May 2021)
Pandey, V. C., and Kumar, A. (2013). Leucaena leucocephala: an underutilized plant for pulp and paper production. Genet. Resour. Crop Evol. 60, 1165–1171. doi: 10.1007/s10722-012-9945-0
Parrotta, J. A. (1992). Leucaena leucocephala (Lam.) de Wit. Leucaena, tantan. Leguminosae (Mimosoideae) Legume family (SO-ITF-SM; 52). New Orleans, LA: USDA Forest Service, Southern Forest Experiment Station, Institute of Tropical Forestry. p. 1–8.
Parvin, R., Shapla, T. L., and Amin, M. H. A. (2011). Effect of leaf and Leucaena leucocephala different tree depth soil on the allelopathy of agricultural crops. J. Innovat. Dev. Strat. 5, 61–69.
Pascal, N., and Salvator, K. (1994). Leucaena psyllid in Burundi. In: Workshop Proceedings, Leucaena psyllid: a threat to agroforestry in Africa, Country Reports. Dar-es-Salaam, United Republic of Tanzania: Tanzania Forest Research Institute. http://www.fao.org/3/v5020e/V5020E05.htm#04.1 (accessed May 28, 2021).
Paynter, Q., and Dodd, S. (2012). Weed Control Scoping Study in the Cook Islands. Auckland, New Zealand: Landcare Research. Available online at: https://www.sprep.org/attachments/VirLib/CookIslands/weed-biocontrol-scoping-study.pdf (accessed May 28, 2021)
Peng, S. H., Wang, H. H., and Kuo, Y. L. (2019). Methods for preventing the invasion of Leucaena leucocephala in coastal forests of the Hengchun Peninsula, Taiwan. Taiwan J. For. Sci. 34, 99–112.
Raghu, S., Wiltshire, C., and Dhileepan, K. (2005). Intensity of pre-dispersal seed predation in the invasive legume Leucaena leucocephala is limited by the duration of pod retention. Austral Ecol. 30, 310–318. doi: 10.1111/j.1442-9993.2005.01475.x
Ramírez-Bahena, M. H., Flores-Félix, J. D., Velázquez, E., and Peix, Á. (2020). The Mimosoid tree Leucaena leucocephala can be nodulated by the symbiovar genistearum of Bradyrhizobium canariense. Syst. Appl. Microbiol. 43, 126041. doi: 10.1016/j.syapm.2019.126041
Rawanawshah, T. (1994). “The Leucaena psyllid in Mauritius”, in Workshop Proceedings, Leucaena psyllid: a threat to agroforestry in Africa, Country Reports. Dar-es-Salaam, United Republic of Tanzania: Tanzania Forest Research Institute. Available online at: http://www.fao.org/3/v5020e/V5020E05.htm#04.1 (accessed May 28, 2021).
Richardson, D. M., and Rejmánek, M. (2011). Trees and shrubs as invasive alien species–a global review. Divers. Distrib. 17, 788–809. doi: 10.1111/j.1472-4642.2011.00782.x
Ruiz, T. E., Febles, G. J., Castillo, E., Simón, L., Lamela, L., Hernández, I., et al. (2019). Leucaena feeding systems in Cuba. Trop. Grassl. 7, 403–406. doi: 10.17138/TGFT(7)403-406
Sanabria-Silva, A. M., and Amarillo-Suárez, Á. R. (2017). Same but different: diversity and complexity of an arthropod trophic network and comparative seed viability of an invasive and a native legume species. J. Arid Environm. 145, 10–17. doi: 10.1016/j.jaridenv.2017.04.004
Sand, P. H. (2009). Diego Garcia legal black hole—a response to Sheppard et al. J. Environ. Law. 21, 295–298. doi: 10.1093/jel/eqp014
Sartori, R. A., Martins, G. A. C., Za,ú, A. S, and Brasil, L. S. C. (2019). Urban afforestation and favela: a study in a community of Rio de Janeiro, Brazil. Urban For. Urban Green. 40, 84–92. doi: 10.1016/j.ufug.2018.10.004
Sekar, K. C. (2012). Invasive alien plants of Indian Himalayan region—diversity and implication. Am. J. Plant Sci. 3, 17533. doi: 10.4236/ajps.2012.32021
Senan, A. S., Tomasetto, F., Farcomeni, A., Somashekar, R. K., and Attorre, F. (2012). Determinants of plant species invasions in an arid island: evidence from Socotra Island (Yemen). Plant Ecol. 213, 1381–1392. doi: 10.1007/s11258-012-0098-1
Sharratt, M., and Olckers, T. (2019). Responses of the seed-feeding beetle Acanthoscelides macrophthalmus and its recruited parasitoids to resource availability–Implications for the biological control of Leucaena leucocephala in South Africa. Biol. Control. 135, 102–109. doi: 10.1016/j.biocontrol.2019.05.013
Sharratt, M. E. J., and Olckers, T. (2012). The biological control agent Acanthoscelides macrophthalmus (Chrysomelidae: Bruchinae) inflicts moderate levels of seed damage on its target, the invasive tree Leucaena leucocephala (Fabaceae), in the KwaZulu-Natal coastal region of South Africa. Afr. Entomol. 20, 44–51. doi: 10.4001/003.020.0106
Singh, H. P., Batish, D. R., and Kohli, R. K. (1999). Allelopathic effect of Leucaena leucocephala on Zea mays. J. Tropical For. Sci. 11, 801–808.
Sithole, N., Tsvuura, Z., Kirkman, K., and Magadlela, A. (2021). Nitrogen source preference and growth carbon costs of Leucaena leucocephala (Lam.) de Wit saplings in South African Grassland soils. Plants. 10, 2242. doi: 10.3390/plants10112242
Soedarjo, M., and Borthakur, D. (1996). Simple procedures to remove mimosine from young leaves, pods and seeds of Leucaena leucocephala used as food. Int. J. Food Sci. 31, 97–103. doi: 10.1111/j.1365-2621.1996.24-321.x
Soedarjo, M., and Borthakur, D. (1998). Mimosine, a toxin produced by the tree-legume Leucaena provides a nodulation competition advantage to mimosine-degrading Rhizobium strains. Soil Biol. Biochem. 30, 1605–1613. doi: 10.1016/S0038-0717(97)00180-6
Ssenku, J. E., Ntale, M., Backeus, I., and Oryem-Origa, H. (2017). “Phytoremediation potential of Leucaena leucocephala (Lam.) de Wit. for heavy metal-polluted and heavy metal-degraded environments,” in Phytoremediation Potential of Bioenergy Plants, Bauddh, K., Singh, B., and Korstad, J., (eds.). Springer, Singapore, pp. 189–209
Te Beest, M., Le Roux, J. J., Richardson, D. M., Brysting, A. K., Suda, J., Kubešov,á, M., et al. (2012). The more the better? The role of polyploidy in facilitating plant invasions. Ann. Bot. 109, 19–45. doi: 10.1093/aob/mcr277
Teunissen, E., Ubaitoi, I., and Powell, M. H. (1995). Trees and shrubs for agroforestry on atolls. Agroforestry for the Pacific Technologies: Agroforestry Information Service. Available online at: https://winrock.org/factnet-a-lasting-impact/trees-and-shrubs-for-agroforestry-on-atolls/ (accessed May 28, 2021).
Thamaga, M. W., Mokoboki, H. K., Sebola, N. A., and Ravhuhali, K. E. (2021). Apparent digestibility and nutritional composition of Leucaena leucocephala (Lam) leaf meal incorporated in the diets of Black Australorp and Potchefstroom Koekoek chicken breeds. Trop. Anim. Health Prod. 53, 458. doi: 10.1007/s11250-021-02922-w
Thaman, R. R., Hassall, D. C., and Takeda, S. (2009). The Vegetation and Flora of Nauru – 2007: Current Status, Cultural Importance and Suggestions for Conservation, Restoration, Rehabilitation, Agroforestry and Food, Health and Economic Security. Suva, Fiji Islands: Secretariat of the Pacific Community. Available online at: https://www.sprep.org/attachments/VirLib/Nauru/1.pdf (accessed May 28, 2021).
Timyan, J. (1996). Bwa Yo: Important Trees of Haiti. Washington DC, US: South-East Consortium for International Development.
Tuda, M., Wu, L. H., Tateishi, Y., Niyomdham, C., Buranapanichpan, S., Morimoto, K., et al. (2009). A novel host shift and invaded range of a seed predator, Acanthoscelides macrophthalmus (Coleoptera: Chrysomelidae: Bruchinae), of an invasive weed, Leucaena leucocephala. Entomological Sci. 12, 1–8. doi: 10.1111/j.1479-8298.2009.00297.x
Turbelin, A. J., Malamud, B. D., and Francis, R. A. (2017). Mapping the global state of invasive alien species: patterns of invasion and policy responses. Global Ecol. Biogeogra. 26, 78–92. doi: 10.1111/geb.12517
Ulbrich, R. (2015). Utilization and Distribution of Selected Invasive Alien Species in Germany and Namibia in Comparison to the Areas of Origin. (Dissertation). The University of Namibia. Available online at: https://repository.unam.edu.na/handle/11070/1429 (accessed May 28, 2021).
Verdecia, D. M., Herrera, R. S., Ramírez, J. L., Leonard, I., Bodas, R., Andrés, S., et al. (2020). Effect of age of regrowth, chemical composition and secondary metabolites on the digestibility of Leucaena leucocephala in the Cauto Valley, Cuba. Agrofor. Syst. 94, 1247–1253. doi: 10.1007/s10457-018-0339-y
Vos, P. (2004). “Case studies on the status of invasive woody plant species in the western Indian Ocean: 2. The Comoros Archipelago (Union of the Comoros and Mayotte),” in Forest Health & Biosecurity Working Papers FBS/4-2E. Food and Agriculture Organization of the United Nations. Rome, Italy: Forestry Department. Available online at: http://www.fao.org/forestry/6845-01e58d615e21ce78bf8146b4afa8d54cd.pdf (accessed May 28, 2021).
Walton, C. (2003a). The biology of Australian weeds. 42. Leucaena leucocephala (Lamark) de Wit. Plant Protect. Q. 18, 90–98.
Walton, C. S. (2003b). Luecaena (Luecaena leucocephala) in Queensland. Pest Status Review Series- Land Protection. Brisbane, Australia: Department of Natural Resources and Mines. Available online at: https://www.daf.qld.gov.au/__data/assets/pdf_file/0009/57294/IPA-Leucaena-PSA.pdf (accessed May 28, 2021).
Wan, J. Z., Zhang, Z. X., and Wang, C. J. (2018). Identifying potential distributions of 10 invasive alien trees: implications for conservation management of protected areas. Environm. Monitor. Assessm. 190, 739. doi: 10.1007/s10661-018-7104-6
Wardatun, S., Harahap, Y., Mun'im, A., Saputri, F. C., and Sutandyo, N. (2020). Leucaena leucocephala (Lam.) de Wit seeds: a new potential source of sulfhydryl compounds. Pharmacognosy J. 12, 298–302. doi: 10.5530/pj.2020.12.47
Wei, C. Y., Wang, J. K., Shih, H. C., Wang, H. C., and Kuo, C. C. (2020). Invasive plants facilitated by socioeconomic change harbor vectors of scrub typhus and spotted fever. PLoS Negl. Tropical Dis. 14, e0007519. doi: 10.1371/journal.pntd.0007519
Whistler, W. A. (1994). Botanical inventory of the proposed Tutuila and of units of the national park of American Samoa. Technical Report 87. University of Hawaii at Manoa, Honololu, Hawaii: Cooperative National Park Resources Studies Unit. Available online at: http://manoa.hawaii.edu/hpicesu/techr/087.pdf (accessed on 28 May 2021)
Willsey, T., Kwon, J. A., Reeves, M. K., Amidon, F., and Miller, S. E. (2019). Mariana Islands Forest. Honolulu, United States: U.S. Fish and Wildlife Service, Pacific Islands Fish and Wildlife Office. Available online at: https://www.researchgate.net/profile/M-Reeves/publication/336401645_Mariana_Islands_Forest/links/5db4ba3a4585155e27074f73/Mariana-Islands-Forest.pdf (accessed on 28 May 2021)
Wolfe, B. T., and Van Bloem, S. J. (2012). Subtropical dry forest regeneration in grass-invaded areas of Puerto Rico: understanding why Leucaena leucocephala dominates and native species fail. For. Ecol. Manag. 267, 253–261. doi: 10.1016/j.foreco.2011.12.015
Wu, L. H., Wang, C. P., and Wu, W. J. (2013). Effects of temperature and adult nutrition on the development of Acanthoscelides macrophthalmus, a natural enemy of an invasive tree, Leucaena leucocephala. Biol. Cont. 65, 322–329. doi: 10.1016/j.biocontrol.2013.03.015
Xu, K. W., Penttinen, P., Chen, Y. X., Zou, L., Zhou, T., Zhang, X., et al. (2013). Polyphasic characterization of rhizobia isolated from Leucaena leucocephala from Panxi, China. World J. Microbiol. Biotechnol. 29, 2303–2315. doi: 10.1007/s11274-013-1396-z
Yirgu, A., Gezahgne, A., and Tsega, M. (2015). First report of Acanthoscelides macrophthalmus (Schaeffer) on Leucaena leucocephala (Lam.) de wit in Ethiopia and a preliminary investigation into its impacts. Afr. Entomol. 23, 280–285. doi: 10.4001/003.023.0234
Yoshida, K., and Oka, S. (2004). Invasion of Leucaena leucocephala and its Effects on the Native Plant Community in the Ogasawara (Bonin) Islands1. Weed Technol. 18, 1371–1375. doi: 10.1614/0890-037X(2004)018(1371:IOLLAI)2.0CO:2
Yousif, M. A. I., Wang, Y. R., and Dali, C. (2020). Seed dormancy overcoming and seed coat structure change in Leucaena leucocephala and Acacia nilotica. Forest Sci Technol. 16, 18–25. doi: 10.1080/21580103.2019.1700832
Yusuff, A. S., Lala, M. A., Popoola, L. T., and Adesina, O. A. (2019). Optimization of oil extraction from Leucaena leucocephala seed as an alternative low-grade feedstock for biodiesel production. SN Applied Sci. 1, 357. doi: 10.1007/s42452-019-0364-0
Zarin, M. A., Wan, H. Y., Isha, A., and Armania, N. (2016). Antioxidant, antimicrobial and cytotoxic potential of condensed tannins from Leucaena leucocephala hybrid-Rendang. Food Sci. Hum. Wellness. 5, 65–75. doi: 10.1016/j.fshw.2016.02.001
Keywords: environmental weed, invasive plant species, leucaena (Leucaena leucocephala), weed management, woody invaders
Citation: Sharma P, Kaur A, Batish DR, Kaur S and Chauhan BS (2022) Critical Insights Into the Ecological and Invasive Attributes of Leucaena leucocephala, a Tropical Agroforestry Species. Front. Agron. 4:890992. doi: 10.3389/fagro.2022.890992
Received: 07 March 2022; Accepted: 25 April 2022;
Published: 24 May 2022.
Edited by:
Buddhi Marambe, University of Peradeniya, Sri LankaReviewed by:
Dulal Borthakur, University of Hawaii at Manoa, United StatesAgnieszka Synowiec, University of Agriculture in Krakow, Poland
Copyright © 2022 Sharma, Kaur, Batish, Kaur and Chauhan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bhagirath S. Chauhan, b.chauhan@uq.edu.au
 Padma Sharma
Padma Sharma Amarpreet Kaur
Amarpreet Kaur Daizy R. Batish
Daizy R. Batish Shalinder Kaur
Shalinder Kaur Bhagirath S. Chauhan
Bhagirath S. Chauhan