Diversity patterns and conservation of the Vigna spp. in Mozambique: A comprehensive study
- 1Linking Landscape, Environment, Agriculture and Food (LEAF), Associated Laboratory TERRA, Instituto Superior de Agronomia (ISA), Universidade de Lisboa, Lisboa, Portugal
- 2Forest Research Center (CEF), Associated Laboratory TERRA, Instituto Superior de Agronomia (ISA), Universidade de Lisboa, Lisboa, Portugal
- 3Royal Botanic Gardens, Kew, Richmond, United Kingdom
- 4Department of Biological Sciences, Eduardo Mondlane University, Maputo, Mozambique
- 5Centre for Ecology, Evolution and Environmental Changes (cE3c) and Change–Global Change and Sustainability Institute, Faculdade de Ciências, Universidade de Lisboa, Lisboa, Portugal
Mozambique supports a high diversity of native legume species, including many Crop Wild Relatives (CWRs). Among them, the still understudied genus Vigna is a particularly notable and promising donor of favorable traits for crop improvement. This study aims to provide an updated overview of Vigna CWRs diversity in Mozambique, defining a conservation strategy for priority target taxa and areas. A checklist of Vigna taxa was prepared and using five criteria (taxonomic group, ethnobotanical value, global and regional distributions, and ex situ conservation status), the prioritization of each taxon was determined. The distribution of Vigna native to Mozambique was studied and diversity hotspots were detected; gaps in in situ conservation were analyzed by overlaying species distribution with Mozambique’s Protected Areas Network. Maps predicting the differences between future conditions and baseline values were performed to investigate expected changes in temperature and precipitation in Vigna’s occurrence areas. There are 21 Vigna native taxa occurring in Mozambique, with the Chimanimani Mountains and Mount Gorongosa, as diversity hotspots for the genus. Following the IUCN Red List criteria, 13 taxa are of Least Concern, while the remaining eight are currently Not Evaluated. According to their priority level for further conservation actions, 24% of the taxa are of high priority, 67% of medium priority, and 9% of low priority. The important hotspot of Chimanimani Mountains is among the areas most affected by the predicted future increase in temperature and reduction of rainfall. The obtained distribution and species richness maps, represent a relevant first tool to evaluate and improve the effectiveness of Protected Areas and IPAs of Mozambique for the conservation of Vigna CWRs. The in situ gap analysis showed that 52% of the Vigna taxa are unprotected; this could be overcome by establishing reserves in Vigna diversity centers, considering the different types of habitats to which the different taxa are adapted, and by increasing in situ protection for the high priority ones. The ex situ conservation of Vigna is very limited and storing seed collections of these CWRs, is an essential component in global food security, as some taxa seem suitable as donors of genetic material to increase resistance to pests and diseases, or to drought and salinity. Overall, we provide recommendations for future research, collecting, and management, to conserve Vigna CWR in Mozambique, providing new data for their sustainable use in crop enhancement, as well as proposing measures for future conservation programs.
1. Introduction
Crop Wild Relatives (CWRs) are undomesticated plants, related to crop species that have the potential to contribute genetic material for crop enhancement (Heywood et al., 2007). Considering the potential negative impact of climate change on biodiversity and food security, strategies for the study and conservation of genetic resources in CWRs must be considered a global priority (Maxted et al., 2010). CWRs offer important sources of useful agronomic traits, which could be incorporated into crop breeding programs, including: tolerance to abiotic and biotic stress; nutraceutical characteristics; and significantly improved crop yields (Pimentel et al., 1997; Tanksley and McCouch, 1997; Meilleur and Hodgkin, 2004; Maxted et al., 2010). Considering the often-limited lifespan of resistant cultivars, the demand for wild genetic breeding material is imperative (e.g., Rocha et al., 2021; Mba and Ogbonnaya, 2022; Vincent et al., 2022). Crop improvement is especially important in areas most likely to be affected by climate changes and with poor management practices of agricultural soils, such as in sub-Saharan Africa (SSA), where food security is particularly alarming (Sintayehu, 2018) and agricultural systems are highly dependent on rainfall rather than on manmade irrigation schemes (Thornton and Herrero, 2015). Sustainable crop production also requires efficient soil fertility management, but soils in wide areas of SSA are highly affected by leaching and erosion, leading to depletion of nutrients (Vidigal et al., 2019). Importantly, more efficient use of nitrogen is urgently needed (Yadav et al., 2017). Crop species from the legume family (Fabaceae or Leguminosae), especially the so-called pulse crops (dry beans, chickpeas, and lentils), can fix atmospheric nitrogen, besides having a high nutritional value as a source of protein (Snapp et al., 2018; Brilhante et al., 2021). African legume species include many CWRs as well as many underutilized locally-adapted crops; thus, promoting and enhancing the productivity of local legume crops will be a valuable contribution to the sustainability of cropping systems and the maintenance of food security in SSA (Vidigal et al., 2019). Many African CWRs are currently threatened, largely due to anthropogenic influences such as habitat loss, unsustainable soil exploitation, and invasive species (e.g., Angola: Catarino et al., 2021a; Malawi: Khaki Mponya et al., 2021; Zambia: Ng’uni et al., 2019). Despite these studies, the CWRs in SSA are still poorly studied, despite being a very rich flora.
Mozambique is recognized as one of the most vulnerable countries to climate change in Africa. More frequent and severe droughts, heatwaves, floods, cyclones, and wildfires are expected in the near future, with a strong negative impact on native flora and food production (INGC, 2009). As an example of climate change effect in Mozambique, it stands out the Cyclone Idai which hit the central area of this country (Sofala) in March 2019 (Charrua et al., 2021a).
A strong candidate genus with high agronomic and food source potential is Vigna Savi (Simon et al., 2007), which includes several widely disseminated crops such as cowpea (Vigna unguiculata). Most taxa of Vigna occur in Africa (Singh, 2020), and ca. 20 are known in Mozambique, where they grow under different ecological conditions (Mackinder et al., 2001; Odorico et al., 2022). In this region, local populations use pulses, particularly of Vigna, for human consumption due to their great protein and iron content, medicinal properties, and as a cheaper mean to enrich the soil with nitrogen (N) (Charrua et al., 2021b). This latter factor is particularly important given that the main factor limiting yield production in this region at present is the lack of N, as a result of the poor agricultural practices as slash-and-burn methods used in tropical and sub-tropical conditions (Simon et al., 2007; Catarino et al., 2021b). The conservation of Vigna CWRs in situ is thus particularly important, but effective conservation of these bioresources requires a detailed knowledge of the species diversity, distribution, and threats, which is lacking in many SSA countries such as Mozambique (Darbyshire et al., 2019).
In this study, we established a comprehensive dataset containing information on the diversity of native Vigna CWRs in Mozambique, as well as georeferenced occurrence records for each taxon. Overall, we aim to provide an updated understanding of the diversity of Vigna CWRs, to contribute new data to support their conservation and sustainable use in Mozambique. Specifically, we intend to: (i) characterize the diversity of the Vigna CWRs occurring in Mozambique, focusing on their taxonomy, distribution, and main uses; (ii) identify the Vigna CWRs taxa prioritary for future conservation measures; and (iii) identify the Vigna CWRs centers of diversity in Mozambique and gaps in in situ and ex situ conservation. Finally, some guidelines to define a conservation strategy for the sustainable use of Vigna CWRs in Mozambique are proposed.
2. Materials and methods
2.1. Study area
Mozambique is located in the south-eastern sector of the African continent with an area of 801,509 km2, bordering Tanzania to the north, the Indian Ocean to the east, Zambia to the northwest, Malawi, and Zimbabwe to the west, and Eswatini and South Africa to the southwest. Its territory is divided into 10 provinces, of which Maputo City, located in the extreme south, encompasses the country’s capital. About 70% of the country is covered by forests or woody vegetation and 26% is included within the network of terrestrial Protected Areas (Ministry for the Coordination of Environmental Affairs, 2014).
Mozambique has a tropical climate in general, with a subtropical climate in the southernmost region. In general, the country has two well-defined seasons: a cold and dry season (from April to October) and a warm and wet season (from October to April) (Barbosa et al., 2001). Due to its great extent, the average annual temperature is differentiated as follows: the northern region with 25.5°C in the coastal region and 18.0°C in the higher regions; followed by the central region with 25.0°C in the coastal region and 20.0°C in the higher interior regions, and finally the southern region with 23.0°C in the coastal region and 25.0°C in the interior region. The average annual rainfall reaches 1,030 mm, ranging from 300 mm/year in the southern region (e.g., lower in Gaza inland toward the border with South Africa and higher along the Maputo coast) to 1,400 mm/year in the Zambezi basin (Uamusse et al., 2017).
The country is considered an important area for plant biodiversity, with high species richness (i.e., about 6,157 native and naturalized plant taxa; Hyde et al., 2022a) and a high level of endemism (Darbyshire et al., 2019), resulting from a great diversity of ecosystems, from dry coastal forests to lowland and montane moist forests, savannas, and mangroves, comprising 13 main ecoregions (Burgess et al., 2004) in which seven plant communities are defined, namely: miombo, mopane, and undifferentiated woodland, Afromontane, halophytic and swamp vegetation, and coastal area (Bandeira et al., 1996).
The geographic distribution of plant richness in Mozambique has been demonstrated in several studies (e.g., Darbyshire et al., 2019; Odorico et al., 2022), which report the following centers of plant endemism: Chimanimani-Nyanga (including the highlands of Manica and Sofala), Mulanje-Namuli-Ribáuè (including the highlands of Nampula and Zambezia), Maputaland [subdivided in three subcentres: Maputaland sensu stricto (including Maputo and Gaza), Lebombo Mountains (in Maputo province only), and Inhambane Center (including Gaza and Inhambane)], and Rovuma (including the coastal area of Cabo Delgado, Nampula, and Zambezia).
2.2. CWRs inventory and data collection
A comprehensive dataset on the native Vigna taxa occurring in Mozambique was collated, which includes the scientific and (where available) common names, taxonomic section, global and regional native distribution, International Union for Conservation of Nature (IUCN) conservation status, and the main uses of the species. These data were obtained by means of a comprehensive review conducted through herbarium collections, relevant publications on Mozambique Flora, and online databases. Therefore, this study was made using three main sources:
(1) Herbarium specimens of Vigna collected in Mozambique from the Herbarium of the Instituto de Investigação Científica Tropical, University of Lisbon (LISC) and Herbarium of the Royal Botanic Gardens, Kew (K) were studied in detail to collect data on the main uses, ecology, life-form, and distribution.
(2) A thorough investigation of native Vigna from Mozambique data described in the literature. The following relevant publications were consulted: Singh et al. (1997), Mackinder et al. (2001), Da Silva et al. (2004), Maxted et al. (2004), Darbyshire et al. (2019), Singh (2020), and Odorico et al. (2022).
(3) Relevant online databases, namely: (i) POWO–Plants of the World Online (POWO, 2022)1 for taxonomic and global distribution data; (ii) Flora of Mozambique (Hyde et al., 2022a)2 for regional distribution data; PROTA–The Plant Resources of Tropical Africa (PROTA, 2022)3 to access the main uses of Vigna species; (iii) IUCN--Red List of Threatened Species (IUCN, 2022)4 to access data on threats and conservation actions; (iv) Genesys Database (Genesys, 2022)5 for ex situ conservation data; (v) “The Crop Wild Relatives Project” (CWR Project, 2022)6 to investigate the Vigna wild species identified as CWR of crop species; and (vi) Global Biodiversity Information Facility (GBIF) (GBIF, 2022a)7 to extract distribution data from sources in addition to the herbaria listed above; and (vii) Germplasm resources information network (GRIN Taxonomy) (USDA, 2022) for data on gene pool, trait type and breeding type.
The scientific names of each species were checked and updated according to Plants of the World Online (POWO, 2022). Moreover, subgenera and sections of Vigna into which each species is placed follow the classification of Maxted et al. (2004).
2.3. CWR prioritization
The conservation of CWRs is essential to guarantee the existence of useful genetic resources to supplement the breeding pool of cultivar species. Therefore, conservation planning is necessary, and prioritization is one of the key steps to developing conservation strategies. Here, based on the collated data, we prioritized the 21 Vigna taxa native to Mozambique based on the criteria described by Magos Brehm et al. (2010), which were selected and adapted to the context of the present study. Therefore, we applied the following five criteria: (i) potential utilization for crop improvement; (ii) ethnobotanical uses; (iii) regional distribution; (iv) global distribution; and (v) ex situ conservation status. Each criterion was applied as follows:
(i) The potential use for crop improvement was based on the concept of the Taxonomic Group (TG) which recognizes the relatedness between crop species and CWRs using the known taxonomic hierarchy. By the application of the TG concept, in decreasing order of affinity with the cultivar, Taxon Group 1 (TG1) includes the cultivar species and other taxa of the same species; Taxon Group 2 (TG2) includes taxa from the same infrageneric section of the cultivar; Taxon Group 3 (TG3) includes taxa of the same subgenus as the cultivar; and Taxon Group 4 (TG4) includes taxa of the same genus as the cultivar (Maxted et al., 2006). We used TG instead of the Gene Pool (GP) concept given that crossbreeding and genetic diversity information was not available for all the studied species. The TG concept was applied based on cowpea (Vigna unguiculata subsp. unguiculata) due to its importance in Mozambique as it constitutes the most important crop in the country. The potential use for crop improvement was then scored as: TG1 (score 4); TG2 (score 3); TG3 (score 2); TG4 (score 1).
(ii) The ethnobotanical value was based on the main traditional uses of Vigna CWRs, with food use as the main focus. Following the criteria defined by Catarino et al. (2021a), we categorized and scored the ethnobotanical value as: used as food (score 4); two or more uses, excluding food (score 3); one use, excluding food (score 2); no recorded uses (score 1).
(iii) The regional distribution was based on the distribution of Vigna CWRs, through the 10 Mozambique provinces. Therefore, we categorized and scored as: one or two provinces (score 4); three, four, or five provinces (score 3); six, seven, or eight provinces (score 2); nine or ten provinces (score 1).
(iv) The global distribution was categorized and scored as: restricted to the neighborhood countries of Mozambique (i.e., Malawi, South Africa, Eswatini, Tanzania, and Zimbabwe) (score 4); restricted to Zambezian Region (score 3); confined to the African continent (score 2); also present outside Africa (score 1). Note that there are no endemic taxa of Vigna in Mozambique (Darbyshire et al., 2019).
(v) The ex situ conservation status considers the number of accessions available in worldwide germplasm banks. Therefore, we categorized and scored this criterion as: zero accessions (score 4); one to four accessions (score 3); five to nine accessions (score 2); more than ten accessions (score 1).
For each Vigna CWR taxon, the scores assigned to each criterion were accumulated, with the total score varying from 5 to 20. Considering the obtained scores, prioritization for conservation was established as follows: high priority (scores between 16 and 20); medium priority (scores between 11 and 15); and low priority (scores between 5 and 10).
2.4. Hotspot diversity and conservation analysis
The geographic distribution of Vigna species native to Mozambique was obtained based on occurrence data extracted from the GBIF databases (GBIF, 2022b), in total 1,014 records. Duplicate records (i.e., records with the same collector and collection number) were excluded and the location of all records was confirmed using Google Earth Pro 7.3.4.8573 (Serea, 2018). The occurrence database was supplemented with data from herbarium specimens, as noted above. The location data obtained through the labels of the herbarium specimens were georeferenced according to the criteria established by Chapman and Wieczorek (2006). The final database contained 295 occurrence records belonging to 21 taxa, which served as the basis for distribution studies and hotspot identification. Species richness maps were constructed in QGIS v.3.4.4 software (QGIS Development Team, 2022) to detect the areas of high diversity of Vigna CWRs in Mozambique (i.e., diversity hotspots). To identify the gaps in the in situ conservation of Vigna CWRs, the areas with high conservation interest were identified based on local species distribution by overlaying the species distribution map with the Protected Areas Network and Important Plant Areas of (IPAs) Mozambique. The geographic boundaries of Mozambique’s Protected Areas were obtained as GIS shapefiles from Protected Planet: The World Database on Protected Areas (WDPA) and World Database on Other Effective Area-based Conservation Measures (WD-OECM) (UNEP-WCMC and IUCN, 2022). Mozambique’s network of Important Plant Areas (hereafter, IPAs) was obtained from Darbyshire et al. (in press).
We produced two maps forecasting the differences between future conditions and the baseline values to analyze the predicted changes in temperature and precipitation in Vigna occurrence areas. Baseline data (1970–2000) were retrieved from the WorldClim database (WorldClim, 2020a). Future mean annual temperature (Bio1) and mean annual precipitation (Bio12) for 2061–2080 were obtained from the Coupled Model Intercomparison Project 6 (CMIP6) (Eyring et al., 2016), also available at the WorldClim website (WorldClim, 2020b). The predicted values of future annual temperature and precipitation were obtained as the mean values of the 21 climate models available for the years 2061–2080 (namely, ACCESS-ESM1-5, BCC-CSM2-MR, CanESM5, CanESM5-CanOE, CMCC-ESM2, CNRM-CM6-1, CNRM-CM6-1-HR, CNRM-ESM2-1, EC-Earth3-Veg, EC-Earth3-Veg-LR, GFDL-ESM4, GISS-E2-1-G, INM-CM4-8, INM-CM5-0, IPSL-CM6a-LR, MIROC6, MIROC-ES2L, MPI-ESM1-2-HR, MPI-ESM1-2-LR, MRI-ESM2-0, and UKESM1-0-LL). The data were selected from the Shared Socio-economic Pathway 3-70 (SSP 3-7.0) since this scenario is in the middle of the range [more details in Hausfather (2019)]. The amplitude of the changes was obtained by subtracting the value of baseline temperature and precipitation from future values using QGIS v.3.4.4 software (QGIS Development Team, 2022).
Regarding the ex situ conservation, the total number of Vigna CWRs accessions collected in Mozambique were accessed through the Genesys Database (Genesys, 2022). Thus, the representativeness of Mozambique’s Vigna genetic resources in worldwide Genebanks was assessed.
3. Results
3.1. Diversity of Vigna CWRs in Mozambique
Our results revealed the occurrence of 21 Vigna native taxa in Mozambique, including 13 species, six subspecies, and two varieties (Table 1). Among the 21 taxa, most are perennial climbing herbs (eleven taxa), followed by perennial non-climbing herbs (nine taxa) and only one annual non-climbing herb, V. reticulata.
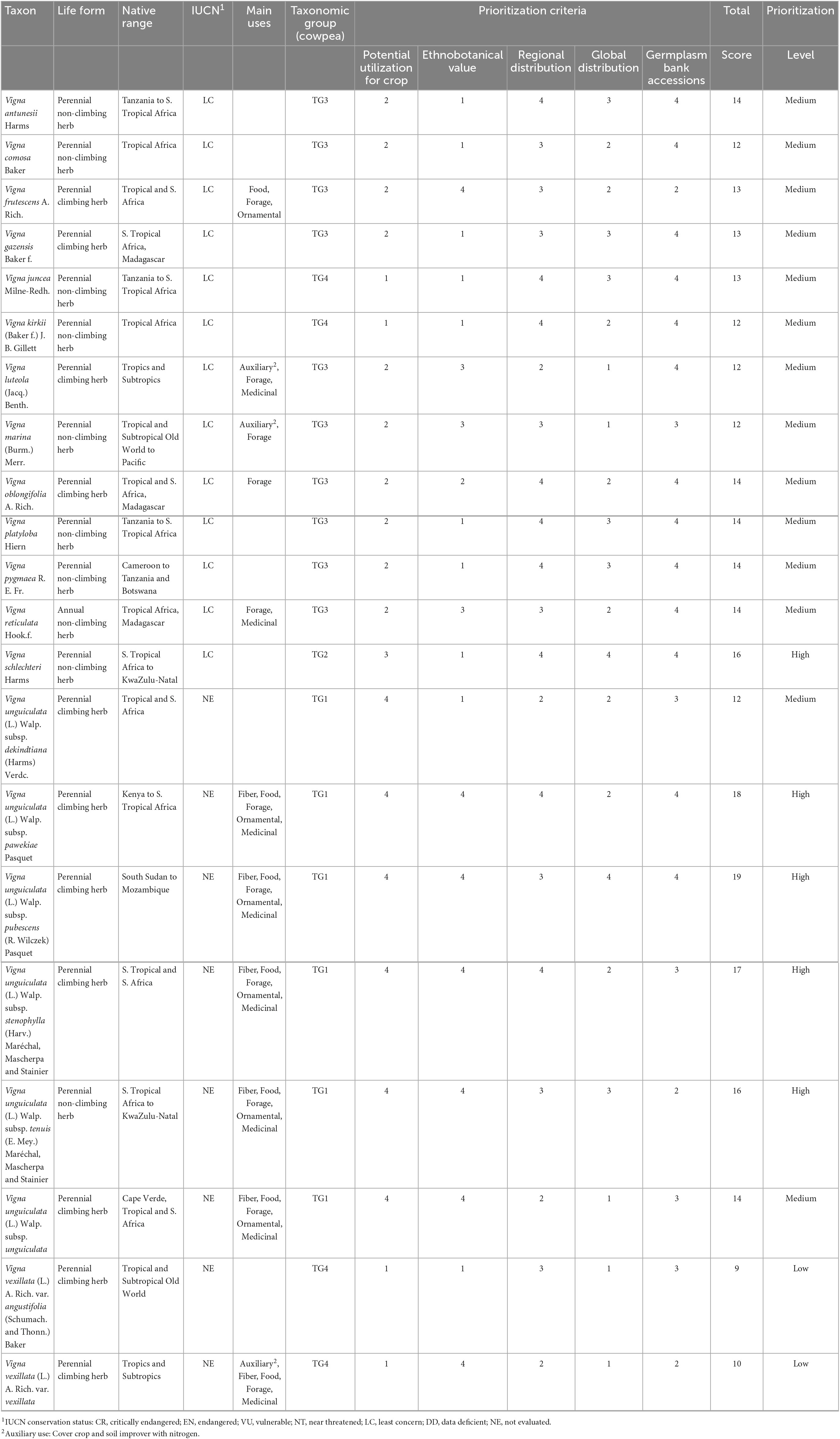
Table 1. Scientific name, life form, regional and global native distribution, International Union for Conservation of Nature (IUCN) conservation status, main uses, cowpea taxonomic groups, scoring of the prioritization criteria and conservation priority level of native Vigna from Mozambique.
Most taxa of Vigna occurring in Mozambique (ca. 80%) are confined to the African continent; the exceptions are V. luteola, V. marina, V. vexillata var. angustifolia, and V. vexillata var. vexillata, which have wider distributions outside Africa.
The information gathered from the IUCN Red List indicated that 62% of those taxa were already evaluated according to the IUCN criteria and categories, with 13 of them classified as of Least Concern; eight taxa remain unevaluated (Table 1).
Regarding the infrageneric taxonomic classification of Vigna, three subgenera (Haydonia, Plectotropis, and Vigna) and seven sections (Catiang, Comosae, Haydonia, Liebrechtsia, Plectotropis, Reticulatae, and Vigna) are recorded in Mozambique (Figure 1; Table 1). The most diverse subgenus is Vigna, with 18 taxa, followed by Plectotropis with two taxa and Haydonia with one. The most diverse section is Catiang, which includes the six V. unguiculata subspecies and V. schlechteri, whilst section Vigna contains four taxa, Reticulatae and Plectotropis three taxa, and Liebrechtsia two taxa. Sections with only one taxon are Comosae (V. comosa) and Haydonia (V. juncea).
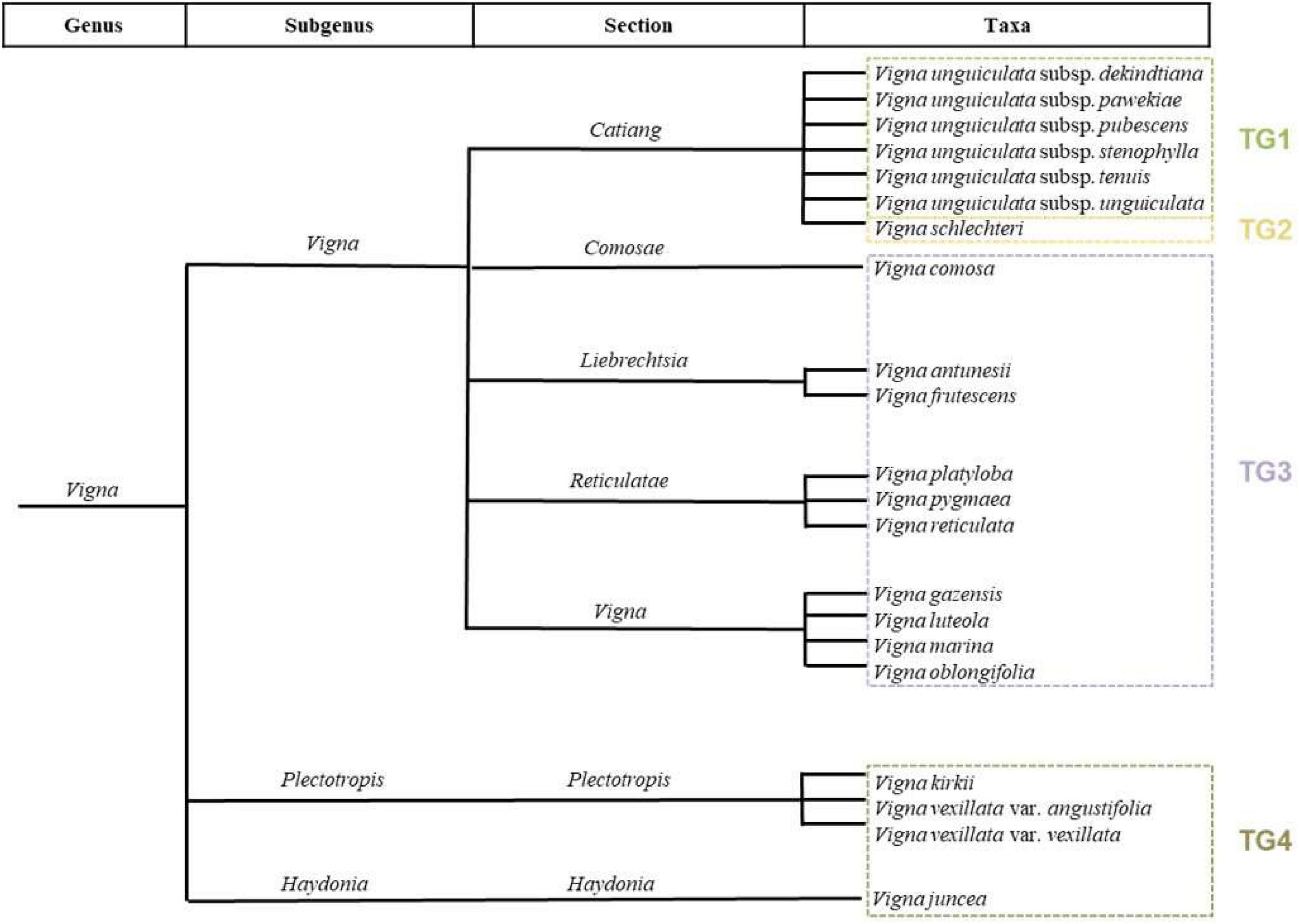
Figure 1. Infrageneric classification of Vigna taxa native to Mozambique standing out subgenus, sections, and taxonomic groups of each taxon.
Through the study of infrageneric categories, it was possible to apply the concept of taxonomic group, which allows to know the proximity between the CWRs taxa and the cultivar.
The results (Figure 1; Table 1) showed that TG1 is composed of six subspecies of V. unguiculata; TG2 is represented by only one species, V. schlechteri; TG3 includes 11 taxa belonging to the same subgenus; and TG4 gathers the remaining three taxa.
Of the 21 taxa, 11 (52%) are used by local human populations (Table 1), each one for various purposes. All the 11 taxa are used as forage, eight as medicines, seven as food, six as fiber, six as ornamental, and three as auxiliary (i.e., cover crop and soil improver with nitrogen). Vigna vexillata var. vexillata is the taxon with most uses (auxiliary, fiber, food, forage, and medicine). Moreover, except for V. unguiculata subsp. dekindtiana, with no recorded uses, the remaining five subspecies of V. unguiculata also share five uses (fiber, food, forage, ornamental, and medicine); V. frutescens and V. luteola have three uses, and V. marina and V. reticulata two uses; Vigna oblongifolia has only one recognized use (forage).
3.2. Priority taxa and in situ conservation gap analysis
Based on prioritization criteria (Table 1), our results revealed that, from the 21 studied taxa, five (24%) are of high priority, 14 (67%) of medium priority, and two (9%) of low priority (Figure 2).
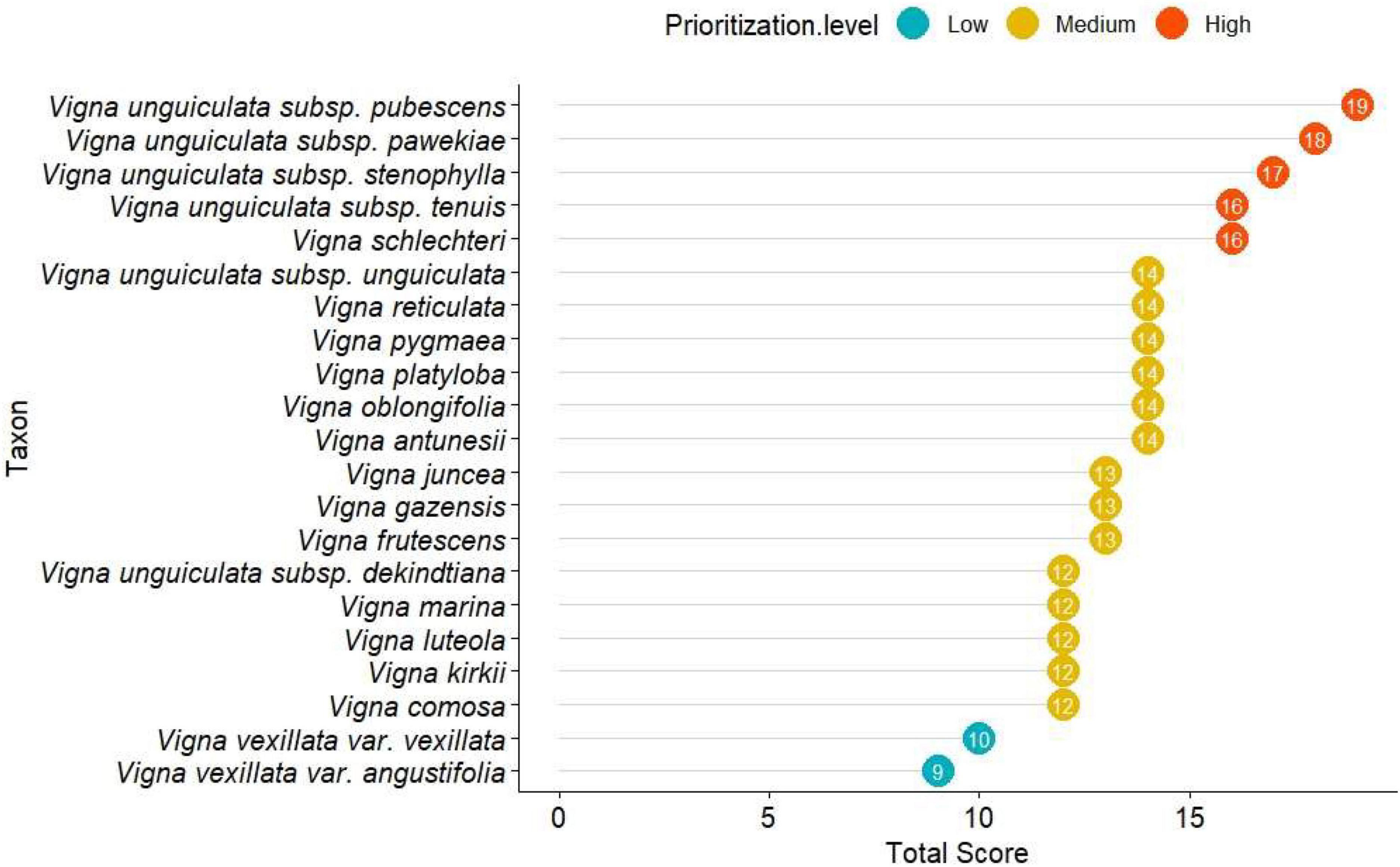
Figure 2. Prioritization scores per Vigna native crop wild relatives (CWRs) to Mozambique, grouped by prioritization level (high, in orange; medium, in yellow; low, in blue). Taxa are listed by decreasing priority score.
Those classified as highly prioritary for conservation are: V. unguiculata subsp. pubescens, V. unguiculata subsp. pawekiae, V. unguiculata subsp. stenophylla, V. schlechteri, V. unguiculata subsp. tenuis, that is, four of the six subspecies of V. unguiculata. Of these, only V. schlechteri, has been evaluated according to the IUCN Red List criteria. Among the 14 taxa considered as of medium priority for conservation, 12 are classified as LC by IUCN, while two remain unevaluated (V. unguiculata subsp. dekindtiana and V. unguiculata subsp. unguiculata). Only two taxa are considered of low priority for conservation, V. vexillata var. vexillata and V. vexillata var. angustifolia, neither of which evaluated for the IUCN Red List. However, they are likely to be of Least Concern since they have a wide distribution, and are not exclusive to the African continent, unlike the other taxa.
The distribution of the genus Vigna in Mozambique was studied based on 295 occurrences corresponding to 21 taxa. A species richness map was constructed and the areas of high diversity in Vigna CWR in Mozambique were identified (i.e., diversity hotspots of the genus). Based on 25 km circular buffers, the number of taxa ranged from 1 to 7, as shown in Figure 3. The diversity hotspots of Vigna genus are in the provinces of Manica, Maputo, Sofala, and Tete. The map (Figure 3) shows six main centers of diversity, namely the Chimanimani Mountains in Manica, and Namaacha in Maputo, with seven taxa each; and Vila Coutinho in Tete, Mount Gorongosa, and Plateau in Sofala, and Manhiça and Inhaca Island in Maputo, all with six taxa. Therefore, the greatest diversity is found in the central and western areas of the country.
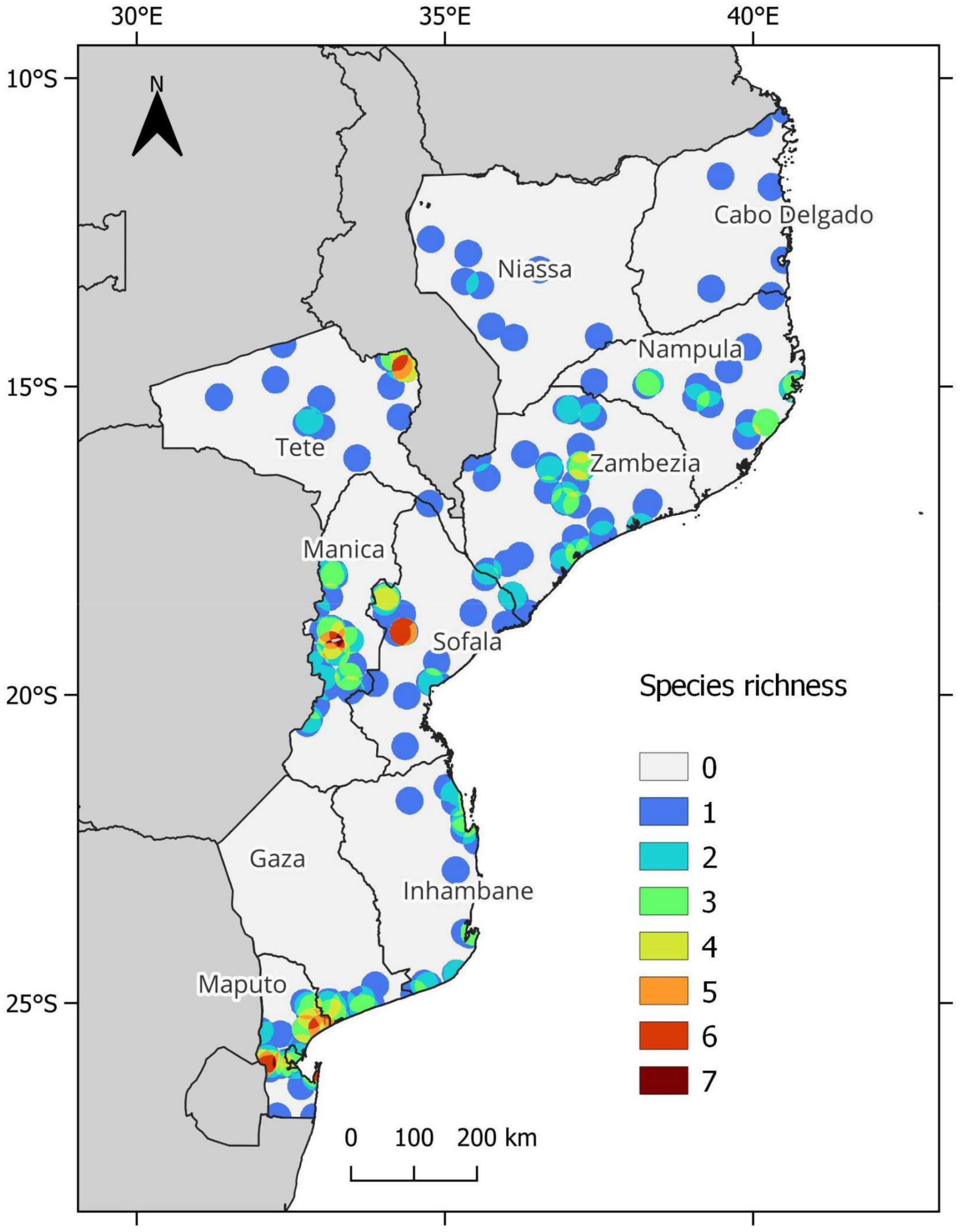
Figure 3. Vigna taxa richness in Mozambique (including subspecies), based on occurrence records by applying a circular buffer of 25 km around each occurrence point.
The maps of predicted changes in temperature (Figure 4A) and precipitation (Figure 4B) for 2061–2080 anticipate the high pressure imposed by climatic changes on Mozambique’s biodiversity. The mean annual temperature will significantly increase, with expected changes ranging from 4.5°C to 6°C. The highest increase is predicted to occur in the western provinces, where the only population of V. schlechteri is found, a species of high priority level. Vigna antunesii and V. juncea, both of medium priority level, also occur only in this area. Two important diversity hotspots, namely Vila Coutinho in Tete, and the Chimanimani Mountains in Manica, are located in areas with a predicted increase of more than 5.5°C. The lowest increase in mean temperature is expected to occur near the coast.
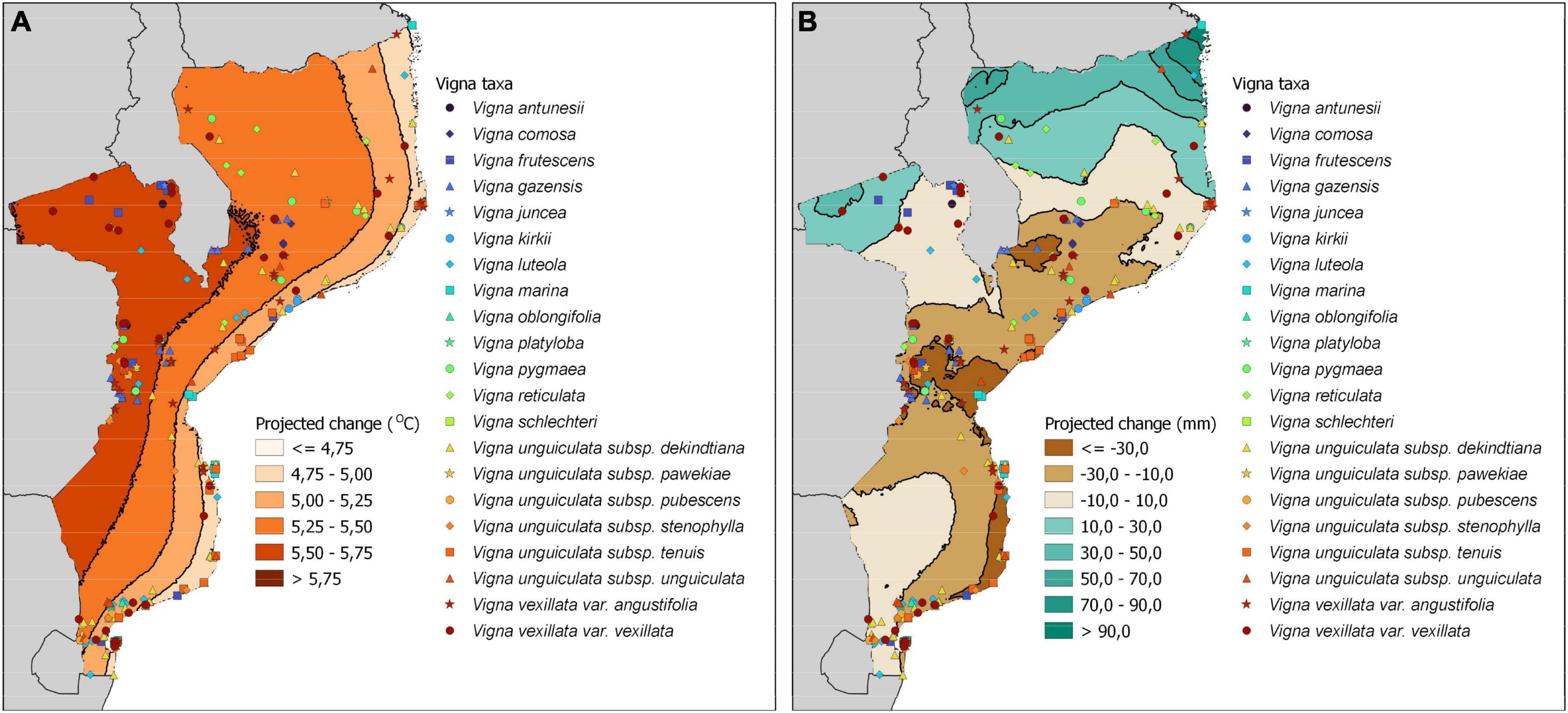
Figure 4. Occurrence of Vigna taxa in Mozambique with details of predicted changes in temperature (A) and precipitation (B) for 2061–2080, obtained with the shared socio-economic pathway 3-70 (SSP 3-7.0).
Changes in precipitation are predicted to increase the annual mean in the north of the country and to decrease it in the central and southeast regions. The important hotspot of Chimanimani Mountains in Manica is among the most affected areas by such a decrease; this region includes all the records of V. unguiculata subsp. pawekiae, a taxon of high priority level. A similar decrease is predicted for the eastern region of Inhambane and Bazaruto Archipelago National Park, affecting many species distributed near the coast.
Approximately 9% of the occurrences of Vigna are within the boundaries of Protected Areas, including 10 (48%) of the 21 studied taxa. On the other hand, 32% of the occurrences are found in IPAs, comprising 12 (57%) of the 21 studied taxa (Figure 5; Table 2). The Gorongosa National Park (including Mount Gorongosa, a portion of the Rift Valley and the southern portion of the Cheringoma Plateau) in the province of Sofala hosts the highest number of taxa (V. gazensis, V. luteola, V. schlechteri, V. unguiculata subsp. dekindtiana, V. unguiculata subsp. pubescens, V. unguiculata subsp. stenophylla, V. vexillata var. vexillata, and V. vexillata var. angustifolia). The Chimanimani National Park (Manica province) includes two taxa (V. gazensis and V. vexillata var. angustifolia); the Bazaruto National Park (Inhambane province) includes two taxa (V. marina and V. unguiculata subsp. tenuis); and the Marromeu National Reserve (Sofala province) includes one single taxon (V. unguiculata subsp. tenuis).
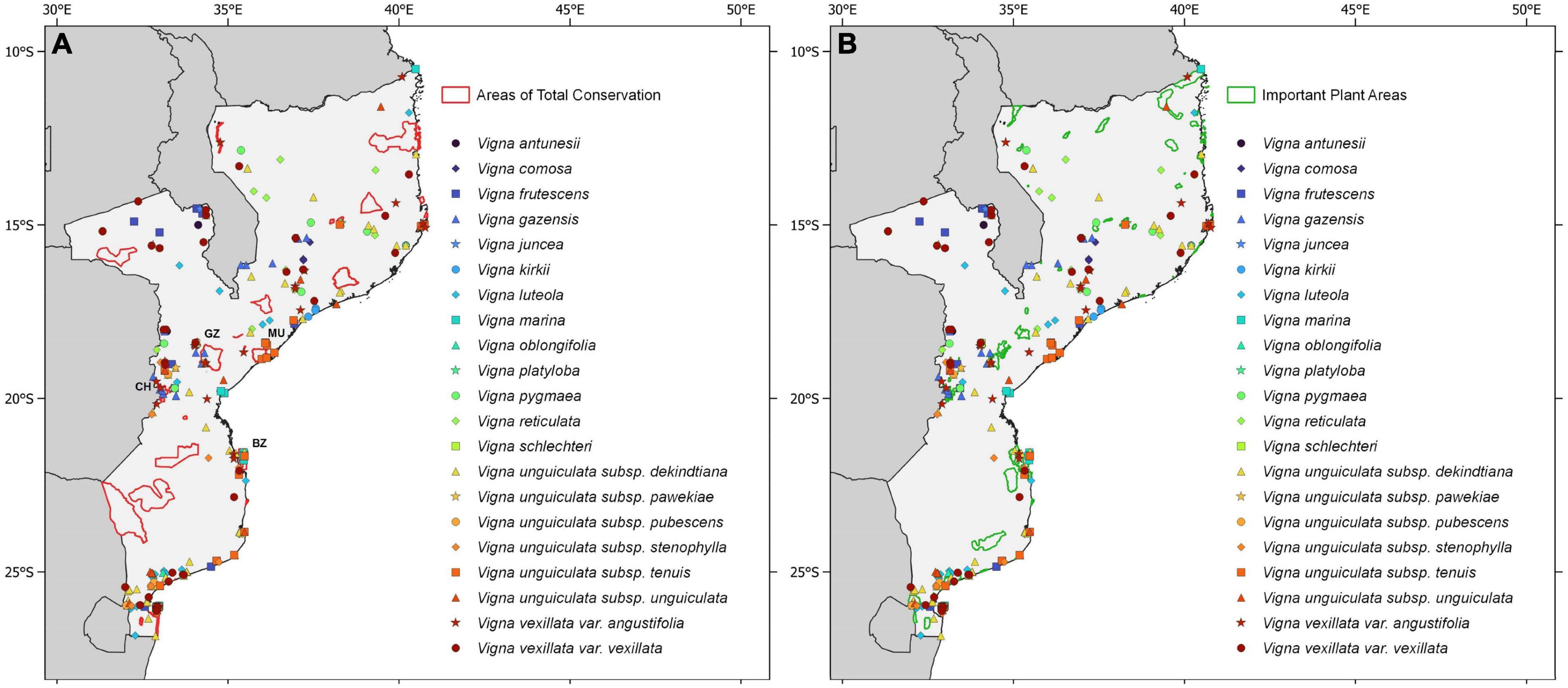
Figure 5. Occurrence of Vigna taxa in Mozambique with details of (A) Protected Areas of total conservation and (B) Important Plant Areas (IPAs). Green lines indicate the IPAs. Red lines indicate the Protected Areas and those with Vigna taxa are highlighted by the respective acronyms [BZ, Bazaruto National Park; CH, Chimanimani National Park; GZ, Gorongosa National (including Mount Gorongosa, a portion of the Rift Valley and the southern portion of the Cheringoma Plateau); MU, Marromeu National Reserve]. Only Protected Areas of total conservation were included, according to Mozambique’s legislation (Diário da República de Moçambique, law no. 205/2017, p.404).

Table 2. Occurrence of Vigna taxa within Protected Areas and Important Plant Areas (IPAs) of Mozambique.
Our results show that all the five taxa categorized as highly prioritary for conservation were found in IPAs, and four of them (V. schlechteri, V. unguiculata subsp. pubescens, V. unguiculata subsp. stenophylla, and V. unguiculata subsp. tenuis) were also found in Protected Areas (Supplementary Figure 1). Of the 14 taxa categorized as of medium priority for conservation, only four were in Protected Areas (V. gazensis, V. luteola, V. marina, and V. unguiculata subsp. dekindtiana); these, together with V. unguiculata subsp. unguiculata, were also found in IPAs (Supplementary Figure 2). The taxa of low priority for conservation (V. vexillata var. angustifolia and V. vexillata var. vexillata) were found in different Protected Areas and IPAs (Supplementary Figure 3).
3.3. Ex situ conservation
The data on the accessions available worldwide revealed that only 30 accessions of native Vigna collected in Mozambique are preserved in germplasm banks (Table 3). Of the 21 studied taxa, only eight (38%) had at least one accession preserved, being represented by fewer than ten accessions each. The taxa V. unguiculata subsp. tenuis and V. vexillata var. vexillata had the highest number of accessions (eight and seven, respectively), followed by V. frutescens with five accessions, V. unguiculata subsp. unguiculata with four accessions, V. marina and V. unguiculata subsp. dekindtiana with two accessions, and V. unguiculata subsp. stenophylla and V. vexillata var. angustifolia with one accession each.
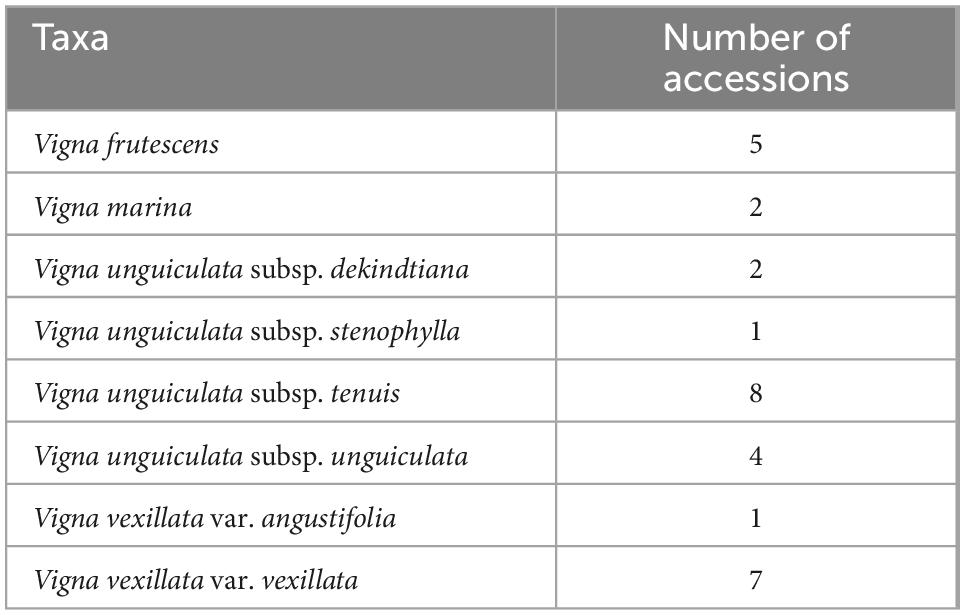
Table 3. Available accessions in worldwide germplasm banks of Vigna Crop Wild Relative (CWR) native in Mozambique, assessed through the Genesys Database (Genesys, 2022).
Most of the 30 accessions are stored at the International Institute of Tropical Agriculture in Nigeria (33%), the rest being at Meise Botanic Garden in Belgium (23%), Centro Internacional de Agricultura Tropical in Colombia (17%), Australian Grains Genebank in Australia (17%), Australian Pasture Genebank in Australia (7%), and International Livestock Research Institute in Ethiopia (3%) (Supplementary Table 1). Notably, Mozambique national germplasm banks lack registered native Vigna accessions.
4. Discussion
4.1. Diversity of Vigna CWRs in Mozambique
The present study analyzed and detailed for the first time the diversity of the genus Vigna in Mozambique, showing that 21 native Vigna taxa occur in this country, with no endemic taxa being recorded. Hyde et al. (2022a) had only referred 19 Vigna taxa recorded for Mozambique, but the present study adds V. oblongifolia and V. unguiculata subsp. dekindtiana to the list, which is consistent with the new checklist of the plants of Mozambique (Odorico et al., 2022) and Maxted et al. (2004), respectively. In comparison to other surrounding African countries, where the occurrence of Vigna is recorded, Mozambique is surpassed by Tanzania (POWO, 2022), Zambia (Bingham et al., 2022), Angola (Catarino et al., 2021a), and Malawi (Hyde et al., 2022b) that have 44, 30, 28, and 24 taxa, respectively. On the other hand, with lower Vigna taxa richness, only Zimbabwe (Hyde et al., 2022c) and Botswana (Hyde et al., 2022d) have 19 and nine taxa, respectively.
The provinces with more taxa are Maputo, Manica, Zambezia, and Sofala, with more than 2,000 taxa recorded from different families. Except for Zambezia, these provinces are also among those with the highest number of occurrences of native Vigna taxa in Mozambique, unevenly distributed across the country. Specifically, the Chimanimani Mountains (Manica), Mount Gorongosa and Plateau (Sofala), Inhaca island (Maputo), Manhiça (Maputo), Namaacha (Maputo), and Vila Coutinho (Tete) are the centers of diversity for the Vigna genus in Mozambique. In this sense, Gorongosa National Park (including Mount Gorongosa, a portion of the Rift Valley and the southern portion of the Cheringoma Plateau) and Chimanimani Mountains were identified as two of the main centers of diversity for Mozambique flora (e.g., Darbyshire et al., 2019; Odorico et al., 2022). Gorongosa National Park, crossed by the Urema rift, is a site of unique characteristics. The climatic, geological, topographic, and edaphic framework promotes an enormous diversity of different plant communities, with woodlands and grasslands standing out as the main ecosystems (Coates-Palgrave et al., 2007). The Chimanimani Mountains are located on the border between Mozambique and Zimbabwe, on the Great Escarpment of Africa (Clark et al., 2017). These are part of the Eastern Afromontane biodiversity hotspot of Critical Ecosystem Partnership Fund (CEPF; Timberlake et al., 2016) and are also considered a subcenter of endemism associated with the larger Chimanimani-Nyanga Center (Van Wyk and Smith, 2001). In addition, it is where the highest number of endemics in any one site in southern tropical Africa is found (e.g., Wursten et al., 2017), being considered an IPA (Darbyshire et al., 2019). The topography and local geology determine the presence of a high number of plant species, most of which restricted to soils derived from quartzite sandstones that are very poor in nutrients (e.g., deficient in phosphorus) (Wursten et al., 2017; Cheek et al., 2018).
It should be noted that the patterns of distribution and species richness maps presented in this study may be spatially biased due to uneven botanical collection effort, as shown by several studies (e.g., Romeiras et al., 2014). A high floristic diversity is recognized in Mozambique; however, although a lot more studies have been carried out since 2014, there are still significant gaps of knowledge about Mozambique’s flora (especially concerning the geographic coverage), but Flora Zambesiaca is now over 90% complete, and the recent work on IPAs (Darbyshire et al., 2019) and associated expansion of the Red List (IUCN, 2020) have resulted in major advances in the current knowledge. Historically, the botanical expedition to Mozambique that allowed the first major advance in the knowledge of local flora took place between 1942 and 1948, when 7,600 herbarium specimens were collected and several plant species were described (Conde et al., 2014). Furthermore, the period from 1963 to 1973 is added to the aforementioned dates, which was also recognized as very important in terms of botanical missions to Mozambique and, consequently, in increasing the country’s botanical knowledge (Martins and Duarte, 2010). After these expeditions, due to the instability caused by two consecutive wars (i.e., war of independence and civil war), botanical studies in the country only restarted from 2000 onward, resulting in the publication of several works, namely: Da Silva et al. (2004), Harris et al. (2011), Timberlake et al. (2011), Wursten et al. (2017), Burrows et al. (2018), Darbyshire et al. (2019), Darbyshire et al. (2020), and Odorico et al. (2022). Even so, more studies and field surveys, to collect occurrence data for future studies on plant diversity and its conservation, are needed to discover new species in Mozambique (Darbyshire et al., 2019). Also, given that the boundaries of most of the proposed centers of endemism are still not fully ascertained, the contribution of botanical collection would be crucial to completely define them. This will make it possible to generate and improve tools for the sustainable use of genetic resources in Mozambique, namely the Vigna CWRs.
4.2. In situ and ex situ conservation of Vigna CWRs in Mozambique
Our results showed that 48% of the studied taxa occur in the Protected Areas of total conservation (i.e., Vigna gazensis, V. luteola, V. marina, V. schlechteri, V. unguiculata subsp. dekindtiana, V. unguiculata subsp. pubescens, V. unguiculata subsp. stenophylla, V. unguiculata subsp. tenuis, V. vexillata var. angustifolia, and V. vexillata var. vexillata); however, this should be regarded as preliminary information and further field confirmation is needed. It should be noted that one of the high conservation priority taxa, V. unguiculata subsp. pawekiae, is not protected in situ.
The protected area with the highest number of Vigna CWRs taxa (i.e., eight taxa) is Gorongosa Natural Park, considered an important area of plant diversity by several previous studies, housing more than 500 taxa (e.g., Da Silva et al., 2004; Müller et al., 2012; Darbyshire et al., 2019). Moreover, this site holds the highest number of Vigna taxa with conservation priority (three of high and three of medium priority).
The IPAs of Mozambique highlight key sites for protecting plant diversity and rare and threatened species nationally (Darbyshire et al., 2017), and 15 of them are currently known to support 12 taxa of Vigna CWRs, of which five are classified as of medium priority (V. gazensis, V. luteola, V. marina, V. unguiculata subsp. dekindtiana, V. unguiculata subsp. unguiculata) and five as of high priority for conservation (V. unguiculata subsp. pubescens, V. unguiculata subsp. pawekiae, V. unguiculata subsp. stenophylla, V. schlechteri, and V. unguiculata subsp. tenuis).
The coverage of plant diversity in Mozambique’s Protected Areas network has some gaps. This is supported by the fact that less than 50% of Mozambique’s IPAs network coincides with currently Protected Areas (in terms of numbers of sites rather than area). In this respect, IPAs contain more Vigna CWRs than Protected Areas, despite their much smaller total area (less than 3% of Mozambique’s total land area, in contrast with 30%, in terms of Protected Areas), as they are more targeted to plant diversity. In this sense, and in agreement with the presented data, it is recognized that the in situ conservation of CWRs in Protected Areas is scarce (Maxted and Kell, 2009).
One of the diversity hotspots for Vigna CWRs, with seven taxa, is located near Vila Coutinho (Tete province), outside Mozambique’s network of Protected Areas and IPAs, and there is no conservation support. Although there are few studies on the flora of Mozambique that highlight this area as important in terms of floristic richness, according to Odorico et al. (2022) Tete is among the five regions with the highest species richness for plants. Nonetheless, specifically for endemic/near-endemic plants, it is the poorest in terms of number of taxa (Darbyshire et al., 2019). It is also worth noting that Vila Coutinho includes five taxa classified as of medium priority for conservation (V. antunesii, V. frutescens, V. juncea, V. reticulata, and V. unguiculata subsp. dekindtiana). Furthermore, our analyses revealed that this region will be one of the most affected by climate change in a medium to long term, namely by the increase of mean annual temperature.
Although Mozambique’s terrestrial Protected Areas occupy around 30% of its territory (UNEP-WCMC and IUCN, 2022), our data indicate that they are inadequate to protect Mozambique’s native CWRs at present. Therefore, management plans must be applied to ensure the sustainable conservation of CWRs in situ (Maxted et al., 2008). The establishment of genetic reserves located in the diversity centers of the genus Vigna, considering the different types of habitats to which they are adapted, can be an alternative to protect and conserve the diversity of these genetic resources (Dulloo et al., 2008).
The ex situ conservation of plant genetic resources in Mozambique, especially CWRs, has been very limited, with little effort to characterize, assess and preserve this genetic heritage. The number of Vigna accessions in world germplasm banks, whose place of origin was Mozambique, only represents eight of the 21 (38%) taxa native to this region (Genesys, 2022). Similarly, and supporting the weak representation of the genus in germplasm banks, Catarino et al. (2021a), found only one accession of the 28 Vigna CWRs taxa from Angola, preserved in a single European germplasm bank. Furthermore, according to Castañeda-Álvarez et al. (2016), the number of accessions preserved in germplasm banks is unrepresentative of the diversity of CWRs that exist globally. Thus, the inherent genetic diversity of Vigna CWRs is neglected and therefore inaccessible to studies for future crop breeding programmes. This is of particular concern for taxa classified as highly prioritary for conservation. To complement the few collections currently conserved ex situ, additional collections should be considered a priority for underrepresented taxa and those of high conservation concern. Moreover, as recommended in studies such as that by Contreras-Toledo et al. (2019), all of the 21 taxa under study should be considered priorities for collection since they each have less than 50 preserved accessions in germplasm banks at present. The diversity hotspots detected for native Vigna in Mozambique can serve as targeted collection sites to maximize the efficient collection of a high number of taxa, providing the germplasm banks with representative samples of the Vigna genetic diversity of Mozambique. The Vigna diversity hotspots represent crucial areas for conservation actions, including further seed collection and habitat protection. Furthermore, the high priority taxa should receive a particular attention for increased in situ protection, especially given their close relationship with the commercial crops.
4.3. Potential use of Vigna CWRs for crop improvement
Studies on genetic diversity and relationships between Vigna taxa are scarce and, therefore, the Gene Pool (GP) for some of these is unknown. Due to this fact, we applied the concept of Taxonomic Group (TG) as a proxy for GP to assess the potential use for the improvement of the main crop, cowpea (V. unguiculata subsp. unguiculata) (Supplementary Table 2). However, the use of TG has some limitations, as stated by Catarino et al. (2021a), such as the fact that taxonomic relationships are unclear and there is little consensus between the various studies carried out previously for the genus (Maxted et al., 2004; Tomooka et al., 2011; Pasquet and Padulosi, 2013). There is an imperative need to carry out new studies to clarify the phylogenetic relationships between species.
In order to expand the cowpea breeding pool for tolerance to abiotic (e.g., drought and salinity) and biotic (e.g., pest resistance) stresses, the Vigna CWRs of GP/TG 1 and 2 represent the most closely related taxa and, therefore, are the most likely to be used as sources of genetic material associated with beneficial traits for plant breeding (e.g., Maxted et al., 2006). Thus, by studying taxa from different populations and different regions, these CWRs are important sources to evaluate adaptive genetic variation in situ, considering the various ecological conditions they are adapted to (Monteiro et al., 2013).
In Mozambique, the wild Vigna taxa are ecologically diverse, occupying various habitats, from arid to humid areas (Mackinder et al., 2001; Supplementary Table 3). Their growth across a range of environments makes them develop a natural resistance/tolerance to stressful abiotic conditions and guarantees their survival and integrity during their life cycle (e.g., Duque et al., 2013). So, the study of their natural genetic variability in situ would promote their role as potential donors of candidate genes involved in adaptive responses associated with these environments.
Previous studies on Vigna CWR revealed greater resistance to pests and diseases than to drought and salinity. In fact, according to van Zonneveld et al. (2020), potential resistance to pests and diseases was reported for 16 of the 20 taxa studied in the present work; with lower incidence only three taxa (V. luteola, V. marina, and V. vexillata var. vexillata) were reported as having potential of resistance to drought and salinity. Moreover, potential resistance to aphids and pod bugs was reported in V. unguiculata subsp. stenophylla (Timko and Singh, 2008; Badiane et al., 2014) and, to aphids, bruchid, cowpea mottle carmovirus, flower thrip, pod bug, pod borer, and yellow mosaic virus, in V. vexillata (Gomathinayagam et al., 1998; Timko and Singh, 2008; Boukar et al., 2015).
5. Conclusion
To the best of our knowledge, this is the first study focused on crop wild relative species of Vigna in Mozambique. The conservation gap analysis revealed that the main Vigna centers of diversity are located outside Protected Areas and that most of the Mozambican genetic diversity of Vigna is not represented in ex situ conservation programmes. Furthermore, the coverage of wild Vigna germplasm accessions from Mozambique in worldwide germplasm banks is insufficient. A targeted seed collecting programme to support future management and ex situ conservation of plant genetic resources in Mozambique is therefore recommended. Further studies concerning the ecological and genetic diversity of the genus should also be carried out to assess the potential of those species for crop improvement under a climate change scenario (e.g., drought conditions). Overall, our data contribute to the understand the status of Vigna CWR taxa in Mozambique, providing new resources and knowledge for their sustainable use in food crop enhancement as well as for measures for future conservation programmes.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MB and MR: conceptualization. MB, MD, and MR: methodology. MB and SC: formal analysis. MB, SB, and ID: investigation. MB: writing—original draft preparation. MB, SC, ID, SB, MM, MD, and MR: writing—review and editing. SB and MR: supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundação para a Ciência e Tecnologia (FCT) and also by the research unit UID/AGR/04129/2020 (LEAF) through the project “MozDiVigna”. MB was supported by the FCT grant (UI/BD/151188/2021).
Acknowledgments
The authors would like to acknowledge the support provided by Fundação para a Ciência e Tecnologia (FCT) and to the research units: UID/AGR/04129/2020 (LEAF) and UID/BIA/00329/2020 (cE3c) funded by Portuguese National Funds through FCT, Portugal. Also, to all the herbarium collection institutes are acknowledged for sharing research material.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.1057785/full#supplementary-material
Footnotes
- ^ https://powo.science.kew.org
- ^ https://www.mozambiqueflora.com/
- ^ https://www.prota4u.org/
- ^ https://www.iucnredlist.org/
- ^ https://www.genesys-pgr.org/
- ^ https://www.cwrdiversity.org/
- ^ https://www.gbif.org/
References
Badiane, F. A., Diouf, M., and Diouf, D. (2014). “Cowpea,” in Broadening the genetic base of grain legumes, eds M. Singh, I. S. Bisht, and M. Dutta (New Delhi: Springer), 95–114. doi: 10.1007/978-81-322-2023-7_5
Bandeira, S. O., Marconi, L., and Barbosa, F. (1996). “Preliminary study of threatened plants of Mozambique,” in The biodiversity of African plants, eds L. J. G. van der Maesen, X. M. van der Burgt, and J. M. van Medenbach de Rooy (Dordrecht: Springer), 306–309. doi: 10.1007/978-94-009-0285-5_38
Barbosa, F. M., Cuambe, C. C., and Bandeira, S. O. (2001). Status and distribution of mangroves in Mozambique. S. Afr. J. Bot. 67, 393–398. doi: 10.1016/S0254-6299(15)31155-8
Bingham, M. G., Willemen, A., Wursten, B. T., Ballings, P., and Hyde, M. A. (2022). Flora of Zambia: Genus page: Vigna. Available online at: https://www.zambiaflora.com/speciesdata/genus.php?genus_id=776 (accessed November 30, 2022).
Boukar, O., Fatokun, C. A., Roberts, P. A., Abberton, M., Huynh, B. L., Close, T. J., et al. (2015). “Cowpea,” in Grain legumes, ed. A. M. De Ron (New York, NY: Springer), 219–250. doi: 10.1007/978-1-4939-2797-5_7
Brilhante, M., Varela, E., P Essoh, A., Fortes, A., Duarte, M. C., Monteiro, F., et al. (2021). Tackling food insecurity in cabo verde Islands: The nutritional, agricultural and environmental values of the legume species. Foods 10:206. doi: 10.3390/foods10020206
Burgess, N., Hales, J. A., Underwood, E., Dinerstein, E., Olson, D., Itoua, I., et al. (2004). Terrestrial ecoregions of Africa and Madagascar. A conservation assessment. Washington, DC: Island Press.
Burrows, J. E., Burrows, S., Schmidt, E., Lotter, M., and Wilson, E. O. (2018). Trees and shrubs Mozambique. Cape Town: Print Matters Heritage.
Castañeda-Álvarez, N. P., Khoury, C. K., Achicanoy, H. A., Bernau, V., Dempewolf, H., Eastwood, R. J., et al. (2016). Global conservation priorities for crop wild relatives. Nat. Plants 2:16022. doi: 10.1038/nplants.2016.22
Catarino, S., Rangel, J., Darbyshire, I., Costa, E., Duarte, M. C., and Romeiras, M. M. (2021a). Conservation priorities for African Vigna species: Unveiling Angola’s diversity hotspots. Glob. Ecol. Conserv. 25:e01415. doi: 10.1016/j.gecco.2020.e01415
Catarino, S., Brilhante, M., Essoh, A. P., Charrua, A. B., Rangel, J., Roxo, G., et al. (2021b). Exploring physicochemical and cytogenomic diversity of African cowpea and common bean. Sci. Rep. 11:12838. doi: 10.1038/s41598-021-91929-2
Charrua, A. B., Padmanaban, R., Cabral, P., Bandeira, S., and Romeiras, M. M. (2021a). Impacts of the tropical cyclone Idai in Mozambique: A multi-temporal landsat satellite imagery analysis. Remote Sens. 13:201. doi: 10.3390/rs13020201
Charrua, A. B., Havik, P. J., Bandeira, S., Catarino, L., Ribeiro-Barros, A., Cabral, P., et al. (2021b). Food security and nutrition in Mozambique: Comparative study with bean species commercialised in informal markets. Sustainability 13:8839. doi: 10.3390/su13168839
Chapman, A. D., and Wieczorek, J. (2006). Guide to best practices for georeferencing. Copenhagen: Global Biodiversity Information Facility, 1–77.
Cheek, M., Chipanga, H., and Darbyshire, I. (2018). Notes on the plant endemics of the quartzitic slopes of Mt Chimanimani (Mozambique & Zimbabwe), and a new, critically endangered species, Empogona jenniferae (Rubiaceae-Coffeeae). Blumea 63, 87–92. doi: 10.3767/blumea.2018.63.01.08
Clark, V. R., Timberlake, J. R., Hyde, M. A., Mapaura, A., Palgrave, M. C., Wursten, B. T., et al. (2017). A first comprehensive account of floristic diversity and endemism on the Nyanga massif, Manica Highlands (Zimbabwe–Mozambique). Kirkia 19, 1–53.
Coates-Palgrave, M., Van Wyk, A. E., Jordaan, M., White, J. A., and Sweet, P. (2007). A reconnaissance survey of the woody flora and vegetation of the Catapú logging concession, Cheringoma district, Mozambique. Bothalia 37, 57–73. doi: 10.4102/abc.v37i1.303
Conde, P., Figueira, R., Saraiva, S., Catarino, L., Romeiras, M., and Duarte, M. C. (2014). The botanic mission to Mozambique (1942-1948): Contributions to knowledge of the medicinal flora of Mozambique. Hist. Ciênc. Saúde Manguinhos 21, 539–585. doi: 10.1590/S0104-59702014000200007
Contreras-Toledo, A. R., Cortés-Cruz, M., Costich, D. E., de Lourdes Rico-Arce, M., Brehm, J. M., and Maxted, N. (2019). Diversity and conservation priorities of crop wild relatives in Mexico. Plant Genet. Res. 17, 140–150. doi: 10.1017/S1479262118000540
CWR Project (2022). The CWR project. Available online at: https://www.cwrdiversity.org/project/ (accessed March 25, 2022).
Da Silva, M. C., Izidine, S., and Amude, A. B. (2004). A preliminary checklist of the vascular plants of Mozamque. Southern African botanical diversity network report (SABONET) No. 30. Pretoria: SABONET, 192.
Darbyshire, I., Anderson, S., Asatryan, A., Byfield, A., Cheek, M., Clubbe, C., et al. (2017). Important plant areas: Revised selection criteria for a global approach to plant conservation. Biodivers. Conserv. 26, 1767–1800. doi: 10.1007/s10531-017-1336-6
Darbyshire, I., Goyder, D. J., Wood, J. R., and Banze, A. E. (2020). Further new species and records from the coastal dry forests and woodlands of the Rovuma Centre of Endemism. Plant Ecol. Evol. 153, 427–445. doi: 10.5091/plecevo.2020.1727
Darbyshire, I., Richards, S., Osborne, J., Matimele, H., Langa, C., Datizua, C., et al. (in press). The important plant areas of Mozambique. Kew, VI: Royal Botanic Gardens.
Darbyshire, I., Timberlake, J., Osborne, J., Rokni, S., Matimele, H., Langa, C., et al. (2019). The endemic plants of Mozambique: Diversity and conservation status. PhytoKeys 136, 45–96. doi: 10.3897/phytokeys.136.39020
Dulloo, M. E., Labokas, J., Iriondo, J. M., Maxted, N., Lane, A., Laguna, E., et al. (2008). “Genetic reserve location and design,” in Conserving plant genetic diversity in protected areas, eds J. M. Iriondo, N. Maxted, and M. E. Dulloo (Wallingford: CABI), 23–64. doi: 10.1079/9781845932824.0023
Duque, A. S., de Almeida, A. M., da Silva, A. B., da Silva, J. M., Farinha, A. P., Santos, D., et al. (2013). “Abiotic stress responses in plants: Unraveling the complexity of genes and networks to survive”, in Abiotic Stress—Plant Responses and Applications in Agriculture, eds K. Vahdati, and C. Leslie (London: InTech), 49–101. doi: 10.5772/52779
Eyring, V., Bony, S., Meehl, G. A., Senior, C. A., Stevens, B., Stouffer, R. J., et al. (2016). Overview of the coupled model intercomparison project phase 6 (CMIP6) experimental design and organization. Geosci. Model Dev. 9, 1937–1958. doi: 10.5194/gmd-9-1937-2016
Gomathinayagam, P., Rathnaswamy, R., and Ramaswamy, N. M. (1998). Interspecific hybridization between Vigna unguiculata (L.) Walp. and V. vexillata (L.) A. Rich. Through in vitro embryo culture. Euphytica 102, 203–209. doi: 10.1023/A:1018381614098
Harris, T., Darbyshire, I., and Polhill, R. (2011). New species and range extensions from Mt Namuli, Mt Mabu and Mt Chiperone in northern Mozambique. Kew Bull. 66, 241–251. doi: 10.1007/s12225-011-9277-9
Hausfather, Z. (2019). CMIP6: The next generation of climate models explained. Carbon brief. Available online at: https://www.carbonbrief.org/cmip6-the-next-generation-of-climate-models-explained (accessed May 28, 2022).
Heywood, V., Casas, A., Ford-Lloyd, B., Kell, S., and Maxted, N. (2007). Conservation and sustainable use of crop wild relatives. Agric. Ecosyst. Environ. 121, 245–255. doi: 10.1016/j.agee.2006.12.014
Hyde, M. A., Wursten, B. T., Ballings, P., and Coates Palgrave, M. (2022a). Flora of Mozambique: Genus page: Vigna. Available online at: https://www.mozambiqueflora.com/speciesdata/genus.php?genus_id=776 (accessed November 20, 2021).
Hyde, M. A., Wursten, B. T., Ballings, P., and Coates Palgrave, M. (2022b). Flora of Malawi: Genus page: Vigna. Available online at: https://www.malawiflora.com/speciesdata/genus.php?genus_id=776 (accessed November 30, 2022).
Hyde, M. A., Wursten, B. T., Ballings, P., and Coates Palgrave, M. (2022c). Flora of Zimbabwe: Genus page: Vigna. Available online at: https://www.zimbabweflora.co.zw/speciesdata/genus.php?genus_id=776 (accessed November 30, 2022).
Hyde, M. A., Wursten, B. T., Ballings, P., and Coates Palgrave, M. (2022d). Flora of Botswana: Genus page: Vigna. Available online at: https://www.botswanaflora.com/speciesdata/genus.php?genus_id=776 (accessed November 30, 2022).
INGC (2009). Main report: INGC climate change report: Study on the impact of climate change on disaster risk in Mozambique, eds K. Asante, G. Brundrit, P. Epstein, A. Fernandes, M. R. Marques, A. Mavume, et al. (Maputo: INGC).
Khaki Mponya, N., Chanyenga, T., Magos Brehm, J., and Maxted, N. (2021). In situ and ex situ conservation gap analyses of crop wild relatives from Malawi. Genet. Resour. Crop Evol. 68, 759–771. doi: 10.1007/s10722-020-01021-3
Mackinder, B., Pasquet, R., Polhill, R., and Verdcourt, B. (2001). “Leguminosae (Papilionoideae),” in Flora Zambesiaca, Vol. 3(Pt 5), eds G. V. Pope and R. M. Polhill (Kew, VI: Royal Botanic Gardens), 1–261.
Magos Brehm, J., Maxted, N., Martins-Loução, M. A., and Ford-Lloyd, B. V. (2010). New approaches for establishing conservation priorities for socio-economically important plant species. Biodivers. Conserv. 19, 2715–2740. doi: 10.1007/s10531-010-9871-4
Martins, E., and Duarte, M. C. (2010). “Caminhos da botânica tropical nos países lusófonos,” in Viagens e Missões científicas nos Trópicos, eds A. Martins and T. Albino (Lisboa: Instituto de Investigação Científica Tropical), 128–132.
Maxted, N., and Kell, S. P. (2009). Establishment of a global network for the. situ conservation of crop wild relatives: Status and needs. Rome: FAO Commission on Genetic Resources for Food and Agriculture.
Maxted, N., Dulloo, E., Ford-Lloyd, B. V., Iriondo, J. M., and Jarvis, A. (2008). Gap analysis: A tool for complementary genetic conservation assessment. Divers. Distrib. 14, 1018–1030. doi: 10.1111/j.1472-4642.2008.00512.x
Maxted, N., Ford-Lloyd, B. V., Jury, S., Kell, S., and Scholten, M. (2006). Towards a definition of a crop wild relative. Biodivers. Conserv. 15, 2673–2685. doi: 10.1007/s10531-005-5409-6
Maxted, N., Kell, S., Toledo, Á, Dulloo, E., Heywood, V., Hodgkin, T., et al. (2010). A global approach to crop wild relative conservation: Securing the gene pool for food and agriculture. Kew Bull. 65, 561–576. doi: 10.1007/s12225-011-9253-4
Maxted, N., Mabuza-Diamini, P., Moss, H., Padulosi, S., Jarvis, A., and Guarino, L. (2004). An ecogeographic study African Vigna. Systematic and ecogeographic studies of crop Genepools 11. Rome: International Plant Genetic Resources Institute.
Mba, C., and Ogbonnaya, F. C. (2022). “Utilizing plant genetic resources to develop climate resilient crops,” in Agricultural biotechnology, biodiversity and bioresources conservation and utilization, eds O. O. Obembe, E. O. Ekundayo, A. S. Okoli, A. Gidado, C. O. Adetunji, A. B. Ibrahim, et al. (Boca Raton, FL: CRC Press), 373–404. doi: 10.1201/9781003178880-22
Meilleur, B. A., and Hodgkin, T. (2004). In situ conservation of crop wild relatives: Status and trends. Biodivers. Conserv. 13, 663–684. doi: 10.1023/B:BIOC.0000011719.03230.17
Ministry for the Coordination of Environmental Affairs (2014). Fifth national report on the implementation of convention on biological diversity in Mozambique. Maputo: Ministry for the Coordination of Environmental Affairs.
Monteiro, F., Romeiras, M. M., Batista, D., and Duarte, M. C. (2013). Biodiversity assessment of sugar beet species and its wild relatives: Linking ecological data with new genetic approaches. Am. J. Plant Sci. 4, 21–34. doi: 10.4236/ajps.2013.48A003
Müller, T., Mapaura, A., Wursten, B., Chapano, C., Ballings, P., and Wild, R. (2012). Vegetation survey of Mount Gorongosa. Occas. Publ. Biodivers. 23, 1–54.
Ng’uni, D., Munkombwe, G., Mwila, G., Gaisberger, H., Brehm, J. M., Maxted, N., et al. (2019). Spatial analyses of occurrence data of crop wild relatives (CWR) taxa as tools for selection of sites for conservation of priority CWR in Zambia. Plant Genet. Resour. 17, 103–114. doi: 10.1017/S1479262118000497
Odorico, D., Nicosia, E., Datizua, C., Langa, C., Raiva, R., Souane, J., et al. (2022). An updated checklist of Mozambique’s vascular plants. PhytoKeys 189, 61–80. doi: 10.3897/phytokeys.189.75321
Pasquet, R. S., and Padulosi, S. (2013). “Genus vigna and cowpea (V. unguiculata [L.] Walp.) taxonomy: Current status and prospects” in Proceedings of the fifth world cowpea conference on improving livelihoods in the cowpea value chain through advancement in science, Dakar.
Pimentel, D., Wilson, C., McCullum, C., Huang, R., Dwen, P., Flack, J., et al. (1997). Economic and environmental benefits of biodiversity. BioScience 47, 747–757. doi: 10.2307/1313097
POWO (2022). Available online at: http://powo.science.kew.org (accessed October 28, 2021).
PROTA, (2022). Available online at: https://prota.prota4u.org/ (accessed November 10, 2021).
QGIS Development Team (2022). QGIS Geographic information system. Open source geospatial foundation project. Available online at: http://qgis.osgeo.org (accessed April 25, 2022).
Rocha, V., Duarte, M. C., Catarino, S., Duarte, I., and Romeiras, M. M. (2021). Cabo Verde’s Poaceae flora: A reservoir of crop wild relatives diversity for crop improvement. Front. Plant Sci. 47:630217. doi: 10.3389/fpls.2021.630217
Romeiras, M. M., Figueira, R., Duarte, M. C., Beja, P., and Darbyshire, I. (2014). Documenting biogeographical patterns of African timber species using herbarium records: A conservation perspective based on native trees from Angola. PLoS One 9:e103403. doi: 10.1371/journal.pone.0103403
Serea, R. (2018). Google earth pro 7.3.4.8573. Available online at: https://www.neowin.net/news/google-earth-pro-7348573/ (accessed April 15, 2022).
Simon, M. V., Benko-Iseppon, A. M., Resende, L. V., Winter, P., and Kahl, G. (2007). Genetic diversity and phylogenetic relationships in Vigna Savi germplasm revealed by DNA amplification fingerprinting. Genome 50, 538–547. doi: 10.1139/G07-029
Singh, B. (ed.) (2020). Cowpea: The food legume of the 21st century, Vol. 164. New York, NY: John Wiley & Sons.
Singh, B. B., Raj, D. M., Dashiell, K. E., and Jackai, L. E. N. (1997). Advances in cowpea research, copublication of international institute of tropical agriculture (IITA) and Japan international research centre for agricultural sciences (JIRCAS). Ibadan: IITA.
Sintayehu, D. (2018). Impact of climate change on biodiversity and as-sociated key ecosystem services in Africa: A systematic review. Ecosyst. Health Sustain. 4, 225–239. doi: 10.1080/20964129.2018.1530054
Snapp, S., Rahmanian, M., Batello, C., and Calles, T. (2018). Pulse crops for sustainable farms in Sub-Saharan Africa. Rome: Food and Agriculture Organization of the United Nations (FAO). doi: 10.18356/6795bfaf-en
Tanksley, S. D., and McCouch, S. R. (1997). Seed banks and molecular maps: Unlocking genetic potential from the wild. Science 277, 1063–1066. doi: 10.1126/science.277.5329.1063
Thornton, P. K., and Herrero, M. (2015). Adapting to climate change in the mixed crop and livestock farming systems in Sub-Saharan Africa. Nat. Clim. Chang. 5, 830–836. doi: 10.1038/nclimate2754
Timberlake, J. R., Darbyshire, I., Wursten, B., Hadj-Hammou, J., Ballings, P., Mapaura, A., et al. (2016). Chimanimani Mountains: Botany and conservation. Report produced under CEPF Grant, 63512. Kew, VI: Royal Botanic Gardens.
Timberlake, J., Goyder, D., Crawford, F., Burrows, J., Clarke, G. P., Luke, Q., et al. (2011). Coastal dry forests in northern Mozambique. Plant Ecol. Evol. 144, 126–137. doi: 10.5091/plecevo.2011.539
Timko, M. P., and Singh, B. B. (2008). “Cowpea, a multifunctional legume,” in Genomics of tropical crop plants, eds P. H. Moore and R. Ming (New York, NY: Springer), 227–258. doi: 10.1007/978-0-387-71219-2_10
Tomooka, N., Kaga, A., Isemura, T., and Vaughan, D. (2011). Vigna. Wild crop relatives: Genomic and breeding resources, legume crops and forages. Berlin: Springer, 291–311. doi: 10.1007/978-3-642-14387-8_15
Uamusse, M. M., Aljaradin, M., Nilsson, E., and Persson, K. M. (2017). Climate change observations into hydropower in Mozambique. Energy Procedia 138, 592–597. doi: 10.1016/j.egypro.2017.10.165
UNEP-WCMC, and IUCN (2022). Protected planet: The world database on protected areas (WDPA) and world database on other effective area-based conservation measures (WD-OECM). Cambridge: UNEP-WCMC.
USDA, Agricultural Research Service, and National Plant Germplasm System (2022). Germplasm resources information network (GRIN Taxonomy). Beltsville, MD: National Germplasm Resources Laboratory.
Van Wyk, A. E., and Smith, G. F. (2001). Regions of floristic endemism in Southern Africa. Hatfield: Umdaus Press.
van Zonneveld, M., Rakha, M., Chou, Y. Y., Chang, C. H., Yen, J. Y., Schafleitner, R., et al. (2020). Mapping patterns of abiotic and biotic stress resilience uncovers conservation gaps and breeding potential of Vigna wild relatives. Sci. Rep. 10:2111. doi: 10.1038/s41598-020-58646-8
Vidigal, P., Romeiras, M. M., and Monteiro, F. (2019). “Crops diversification and the role of orphan legumes to improve the Sub-Saharan Africa farming systems,” in Sustainable crop production, ed. M. Hasanuzzaman (Rijeka: IntechOpen). doi: 10.5772/intechopen.88076
Vincent, H., Hole, D., and Maxted, N. (2022). Congruence between global crop wild relative hotspots and biodiversity hotspots. Biol. Conserv. 265:109432. doi: 10.1016/j.biocon.2021.109432
WorldClim (2020a). Historical climate data. Available online at: https://www.worldclim.org/data/worldclim21.html (accessed May 19, 2022).
WorldClim (2020b). Future climate data. Available online at: https://www.worldclim.org/data/cmip6/cmip6_clim2.5m.html (accessed May 19, 2022).
Wursten, B., Timberlake, J., and Darbyshire, I. (2017). The Chimanimani Mountains: An updated checklist. Kirkia 19, 70–100.
Keywords: conservation strategies, cowpea, gap analysis, protected areas, East Africa, species richness, crop wild relatives
Citation: Brilhante M, Catarino S, Darbyshire I, Bandeira S, Moldão M, Duarte MC and Romeiras MM (2023) Diversity patterns and conservation of the Vigna spp. in Mozambique: A comprehensive study. Front. Ecol. Evol. 10:1057785. doi: 10.3389/fevo.2022.1057785
Received: 03 October 2022; Accepted: 13 December 2022;
Published: 09 January 2023.
Edited by:
Mohd. Kamran Khan, Selçuk University, TürkiyeReviewed by:
Manuel De La Estrella, University of Córdoba, SpainTofazzal Islam, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Bangladesh
Copyright © 2023 Brilhante, Catarino, Darbyshire, Bandeira, Moldão, Duarte and Romeiras. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria M. Romeiras,  mmromeiras@isa.ulisboa.pt
mmromeiras@isa.ulisboa.pt
 Miguel Brilhante
Miguel Brilhante Sílvia Catarino
Sílvia Catarino Iain Darbyshire
Iain Darbyshire Salomão Bandeira
Salomão Bandeira Margarida Moldão1
Margarida Moldão1  Maria Cristina Duarte
Maria Cristina Duarte Maria M. Romeiras
Maria M. Romeiras