Bioactive components, pharmacological effects, and drug development of traditional herbal medicine Rubus chingii Hu (Fu-Pen-Zi)
- 1The First Affiliated Hospital, Zhejiang Provincial Hospital of Chinese Medicine, School of Pharmaceutical Sciences, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 2Zhejiang Research Institute of Traditional Chinese Medicine Co., Ltd., Hangzhou, China
- 3The Third Affiliated Hospital, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
Rubus chingii Hu (Chinese Raspberry), known as Fu-Pen-Zi in Chinese, a woody perennial plant of the genus Rubus in the Rosaceae family, has specific nutritional and medicinal values, which is considered food-medicine herb in China for thousands of years to treat impotence, premature ejaculation, enuresis, frequent urination, and other diseases. This review aims to summarize recent advances in the bioactive components, pharmacological effects, and drug development and utilization of Rubus chingii Hu, hoping to provide useful support for its further research and clinical application. The bioactive components in Rubus chingii Hu contain mainly terpenoids, flavonoids, alkaloids, phenolic acids, polysaccharides, and steroids. The main pharmacological effects are their anti-oxidant, anti-inflammatory, and anti-tumor capacity on human health. Rubus chingii Hu is a very valuable food-medicine herb. The development of Rubus chingii Hu–related drugs is relatively single, which is limited to traditional Chinese medicine and prescriptions. Therefore, it is vital to pay interest to Rubus chingii Hu and its bioactive components in the future and extend its scientific application.
Introduction
With the improvement of living standards worldwide, the intake of high fat and high sugar food and bad habits such as a sedentary lifestyle, the incidence of metabolic diseases such as hypertension, diabetes, obesity, cardiovascular disease (CVD), and nonalcoholic fatty liver disease (NAFLD) has increased year by year (1, 2). Meanwhile, due to the increase in life expectancy, people have put a stronger focus on anti-aging strategies (3). Therefore, it is of great value to consider some foods and their bioactive components to prevent human diseases. Mediterranean diet recommends a certain amount of berries and fruits every day. Therefore, food-medicine herbs have great development and utilization value.
Rubus chingii Hu (Fu-Pen-Zi), belonging to the genus Rubus in the Rosaceae family, is grown in Zhejiang, Jiangsu, Anhui, Jiangxi, Fujian, Guangxi, and Hubei provinces of China (Figure 1) (4). As a traditional Chinese medicine and “third generation” golden fruit, the ripe fresh fruit is delicious and juicy. It has been recorded in “the catalog of the substances traditionally considered as both food and Chinese medicine” in 2002 (5). There is a lot of fupenzic acid, ellagic acid, salicylic acid, and a large number of vitamins and other nutrients in the young and mature fruits of R. chingii Hu (6). Among 194 species of Rubus in China, the dry immature fruit of R. chingii Hu is the only one selected in the “Chinese Pharmacopoeia” 2015 edition (7, 8). In 2018, Zhejiang Province identified R. chingii Hu as one of the cultivated varieties of the new “eight famous herbals in Zhejiang” (Zhe-Ba-Wei), which has great development value (9).

Figure 1. Plant, flowers, dried fruitlet of R. chingii Hu (Fu-Pen-Zi) and related Chinese medicines. (A) Plant of R. chingii Hu, (B) Flowers, (C) WuziYanzong Pill, and (D) Dried fruitlet (Fu-Pen-Zi).
Due to its high nutritional and medicinal value, R. chingii Hu has been frequently used alone or as a component of traditional Chinese medicine (TCM) formulas for thousands of years to cure enuresis, impotence, spermatorrhea, and other diseases (10). Modern medical studies have shown that R. chingii Hu has several biological and pharmacological properties, such as improving learning and memory ability, delaying aging, anti-inflammatory, anti-tumor, immunomodulatory, and anti-oxidant activities (11–14). Therefore, R. chingii Hu has shown good therapeutic effects in many disease fields and has made great progress in pharmacological benefits, which have great development value. But this food-medicine herb and its bioactive components are still far from their clinical application. This review comprehensively summarizes the latest research advances of R. chingii Hu, including its bioactive components, pharmacological effects, quality control, current situation of drug development, possible future food, and pharmacological trends and prospects and points out the new research direction of R. chingii Hu.
Bioactive components of R.chingii Hu
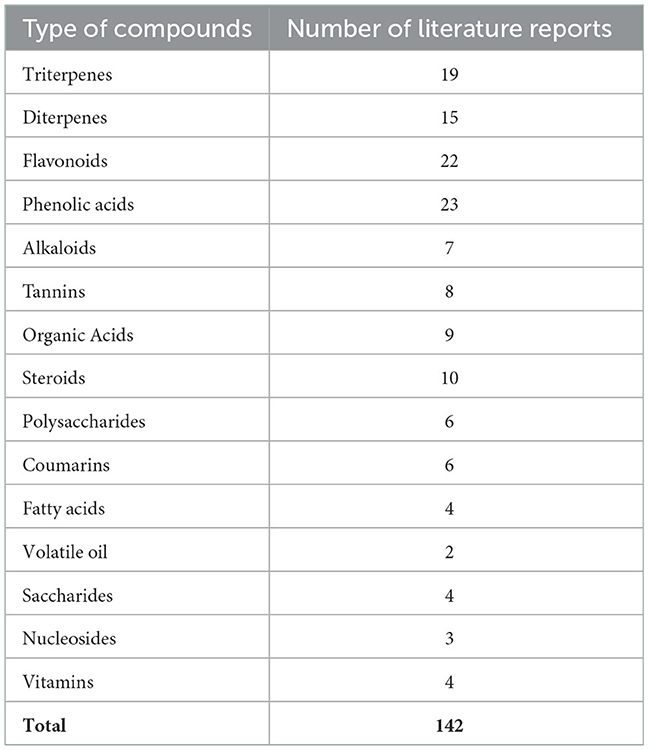
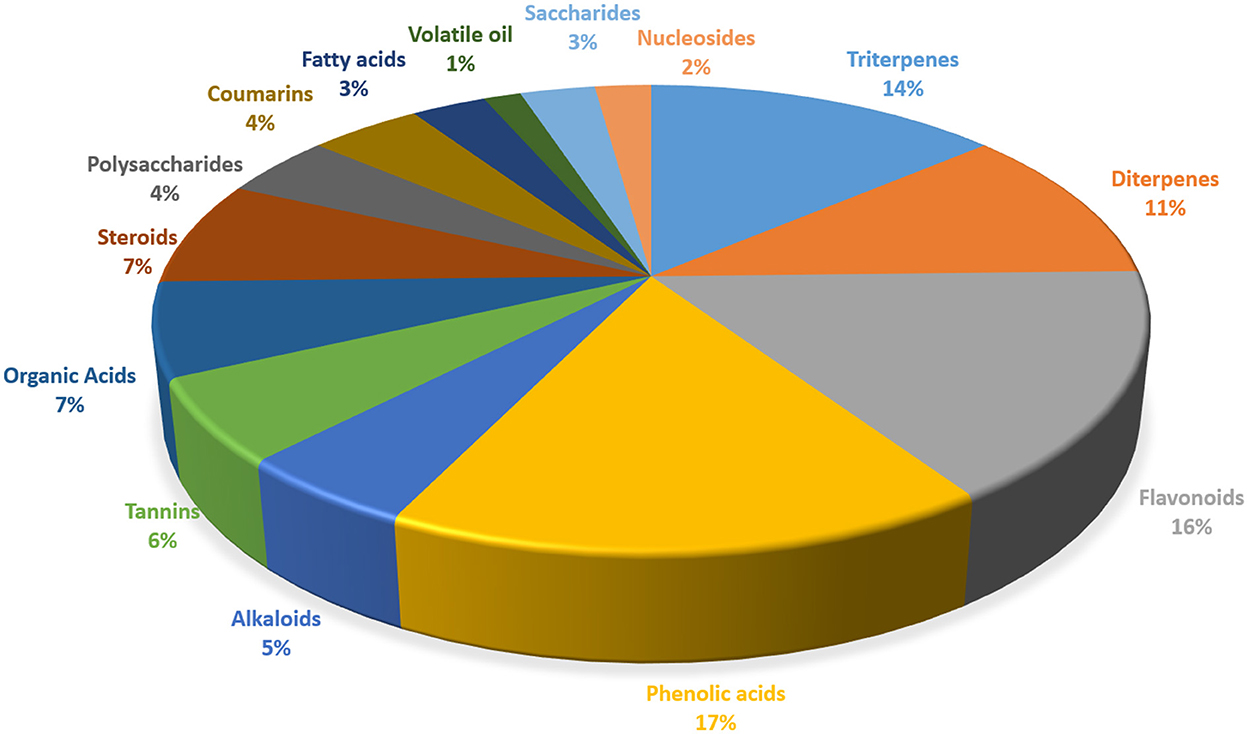
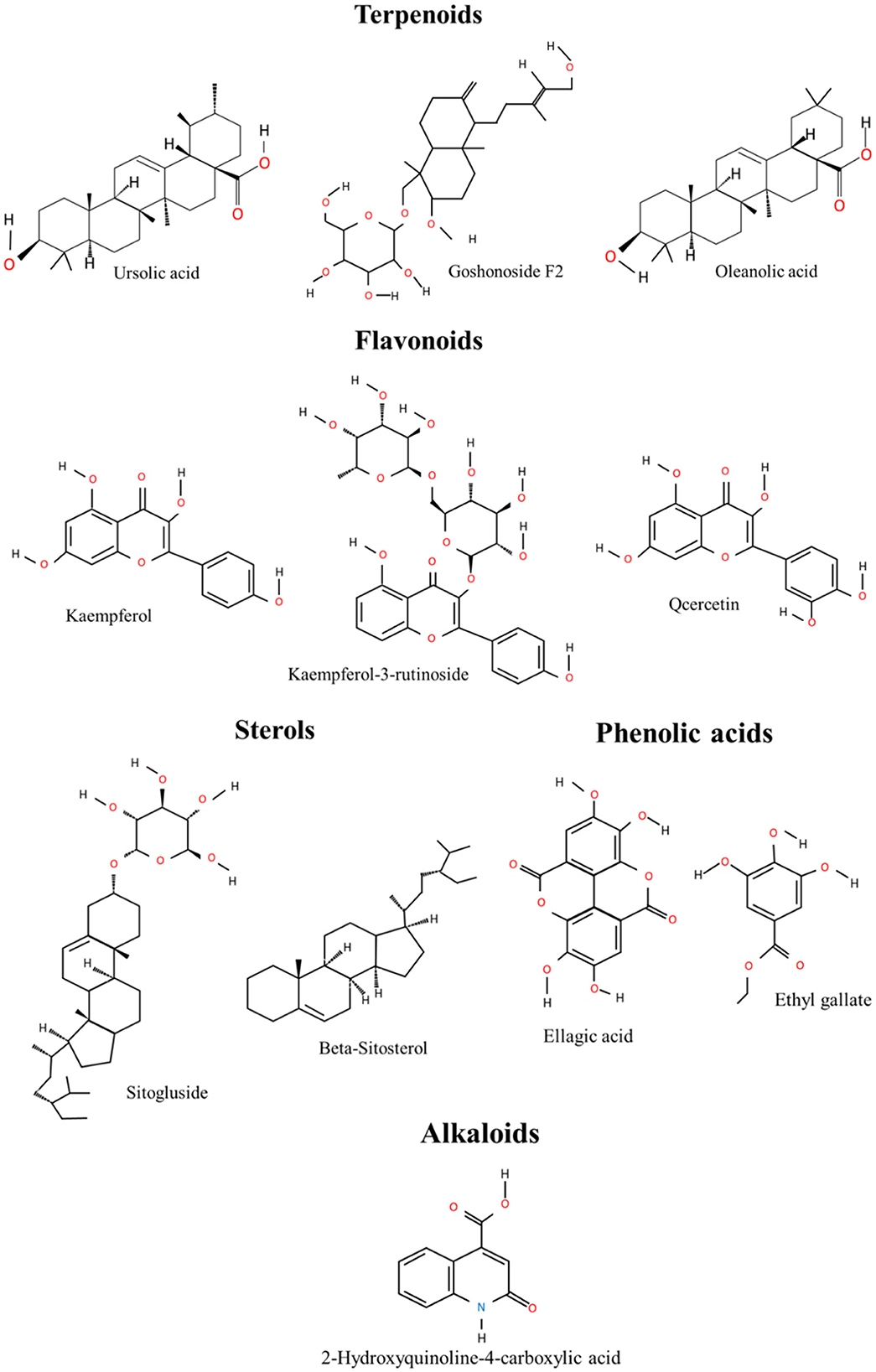
Thus, a multitude of chemical components has been isolated and identified from the leaves and fruits and of R. chingii Hu (Table 1) (6). The main components include terpenoids, flavonoids, alkaloids, phenolic acids, polysaccharides, and sterols. The portion of different components of R. chingii Hu has been shown in Figure 2. The main components' chemical structures are shown in Figure 3.
Terpenoids
Terpenoids are important and typical components of the active components in the genus Rubus. Terpenoids in R. chingii Hu have anti-oxidant, anti-bacterial, anti-cancer, and other physiological activities. According to their structural characteristics, terpenoids are mainly divided into diterpenoids and triterpenoids. The component structure of diterpenes is divided into Labdane type and kaurane type. The representative component of Labdane-type diterpenes is rubusoside, and the ent-16α, 17-dihydroxy-kauran-19-oic acid is the representative of kaurane type (10). Goshonoside is a characteristic diterpene of R. chingii. Goshonoside F1, goshonoside F2, goshonoside F3, goshonoside F4, and goshonoside F5 just exist in leaves (14), while goshonoside G only in fruits (12). Furthermore, goshonoside F6 and goshonoside F7 were extracted from leaves and fruits (13). The triterpenoids from this genus are mainly pentacyclic triterpenoids, which can be divided into Olean and Ursane skeleton structures. Triterpenoids mainly include uronic acid, 2-hydroxyuronic acid, Vlingic acid (tormentic acid), euscaphic acid, hyptatic acid B, rasponic acid, and 2,19,24-trihydroxyurs-urs-12-ene-3-oxo-28-oic acid (15). Therefore, these characteristic components can be used as a reference for R. chingii Hu quality-marker screening.
Flavonoids
The flavonoids in R. chingii Hu have strong anti-oxidant activity and have high value in maintaining human health. They also have anti-inflammatory, anti-aging, and other effects. At present, there are 22 kinds of flavonoids found in R. chingii Hu including quercetin and kaempferol (Table 1). Tiliroside is the first flavonoid isolated from R. chingii Hu. Flavonoids isolated from R. chingii Hu mainly including kaempferol and kaempferol-3-o-β-D-glucopyranoside (astragaloside), kaempferol-3-o-β-Methyld-pyranoglucuronate, kaempferol-3-o-rutoside, kaempferol-3-o-β-D- (6 “-p-hydroxycinnamon Acetyl)-glucoside (lindenin), quercetin-3-o-β-D-glucoside, rutin, phloridin, quercetin, hypericin, and 2” o-galloyl hypericin (16). P-Hydroxyphenyl butanone was found as early as in 1918, not confirmed until 1957 for major flavor components in R. chingii Hu (14). It is one kind of the important raw materials that is not only used in spices and food but also in the fields of medicine, environmental protection, and Chinese cigarettes (10). Due to its very low content and difficulty to separate alone, it is still unable to produce “natural” P-Hydroxyphenyl butanone directly from natural products. Quercetin is one of the most typical and bioactive flavonoids (12). Tiliroside, the most abundant component of flavonoids, has the same tendency as flavonoids (13). Therefore, when measuring the content of flavonoids in plants, the content of tiliroside is always measured. Flavonoids are important polyphenol constituents of R. chingii Hu with various pharmacological effects.
Alkaloids
There are seven kinds of alkaloids found in R. chingii Hu, which are quinoline type, isoquinoline type, and indole type. Alkaloids are a class of active substances found in relatively small amounts in R. chingii Hu (17). They include 4-Hydroxy-2-oxo-1,2,3,4-terahydroquinoline-4-carboxylic acid, methyl 1-oxo-1,2-dihydroisoquinoline-4-carboxylate, 1-oxo-1,2-Dihydroisoquinoline-4-carboxylic acid, Rubusine, Methyl (3-hydroxy-2-oxo-2,3-dihydroindol-3-yl)-acetate, Methyldioxindole-3-acetate, and 2-oxo-1,2-Dihydroquinoline-4-carboxylic acid. Kejun (18) found that the alkaloid 2-hydroxyquinoline-4-carboxylic acid in raspberry has the functions of anti-osteoporosis and phytoestrogens.
Phenolic acids
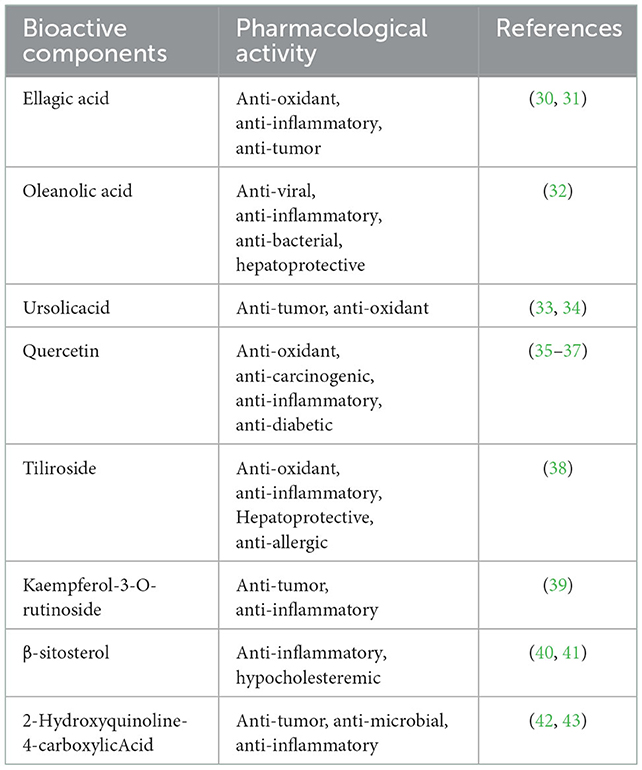
Phenolic acids exist widely in R. chingii Hu. P-hydroxybenzoic acid and ellagic acid are common compounds in this plant. In addition, other phenolic acids were also found in R. chingii Hu mainly including vanillic acid, salicylic acid, ferulic acid, shikimic acid, gallic acid, ethyl gallate, 4-hydroxy-3-methoxybenzaldehyde, p-hydroxybenzaldehyde, and 4-hydroxy-3-methoxybenzoic acid (19). Ellagic acid is a dilactone of polyphenol, a dimer derivative of gallic acid, and belongs to a kind of polyphenol (20).
Steroids
Sterols are a class of compounds with physiological activities, which are widely used in cosmetics, food, and drugs. In R. chingii Hu, steroids are relatively rare, mainly β-sitosterol, daucosterol, and stigmast-4-ene-(3β,6α)-diol (21). β-sitosterol is a compound of steroid with a hydroxyl and a double bond at positions C-3 and C-5, and 10-carbon alkyls on the side chain of C-17 (13). The structure of plant sterols such as carotene and β-sitosterol is similar to that of cholesterol, which can effectively reduce the concentration of cholesterol and low-density lipoprotein in the blood (22). Desai et al. (23) studied the specific mechanism of the lipid-lowering effect of β-sitosterol. In experiments, it was found that β-sitosterol can restore the type 1 cholecystokinin receptor to normal status by competing with cholesterol for binding sites and binding cholecystokinin to promote gallbladder contraction, digestion, and regulate gastric emptying.
Hydrolyzable tannin components
Hydrolyzable Tannins are the most abundant polyphenols in R. chingii Hu that provide health benefits. Up to 20 kinds of HTs were present in leaves, whereas only 10 different HTs were found in the stem (24). Comparative genomic analysis showed that there was a tandem gene cluster of UGT, carboxylesterase, and SCPL genes on chromosome 02 of R. chingii, including 11 CXE, eight UGT, and six SCPL genes, which may be critical for the biosynthesis of HTs. Furthermore, in vitro enzyme assays demonstrated that the proteins encoded by CXE and UGT genes have the functions of tannin hydrolase and gallic acid glycosyltransferase, respectively (24).
Polysaccharide
As an important active ingredient in R. chingii Hu, polysaccharides not only have anti-oxidant, anti-inflammatory, and anti-tumor effects (25) but also have functions such as improving oxidative stress induced by toxic substances (26). Huixia et al. (27) treated the separated crude raspberry polysaccharide by pre-column hydrolysis and derivatization and measured the monosaccharide composition and content of raspberry polysaccharide by HPLC. Raspberry contains glucose, galactose, fructose, mannose, and rat. There are six kinds of monosaccharides such as linose and arabinose, and the ratio of their substances is 0.05:1.37:0.20:1.00:1.95. The monosaccharide components and proportions of each raspberry polysaccharide were different, mainly because the raspberries used in each experiment were produced in different places, and the characters of the raspberries produced were different due to the influence of geography. In addition, R. chingii Hu polysaccharide is rich in dietary fiber, which can also play a role in regulating intestinal prebiotics (26).
Other compounds
The fruits and leaves of R. chingii Hu contain a variety of essential amino acids, trace elements, and sugars, including copper, manganese, zinc, vitamins A, B, C, E, glucose, and fructose (28). Hu et al. compared the composition of essential amino acids and non-essential amino acids in raspberry fruit and leaves and found that the indexes in leaves were close to the indexes stipulated by WHO/FAO, which means that leaves are more high-quality plant protein raw materials (29).
Pharmacological effects of R. chingii Hu
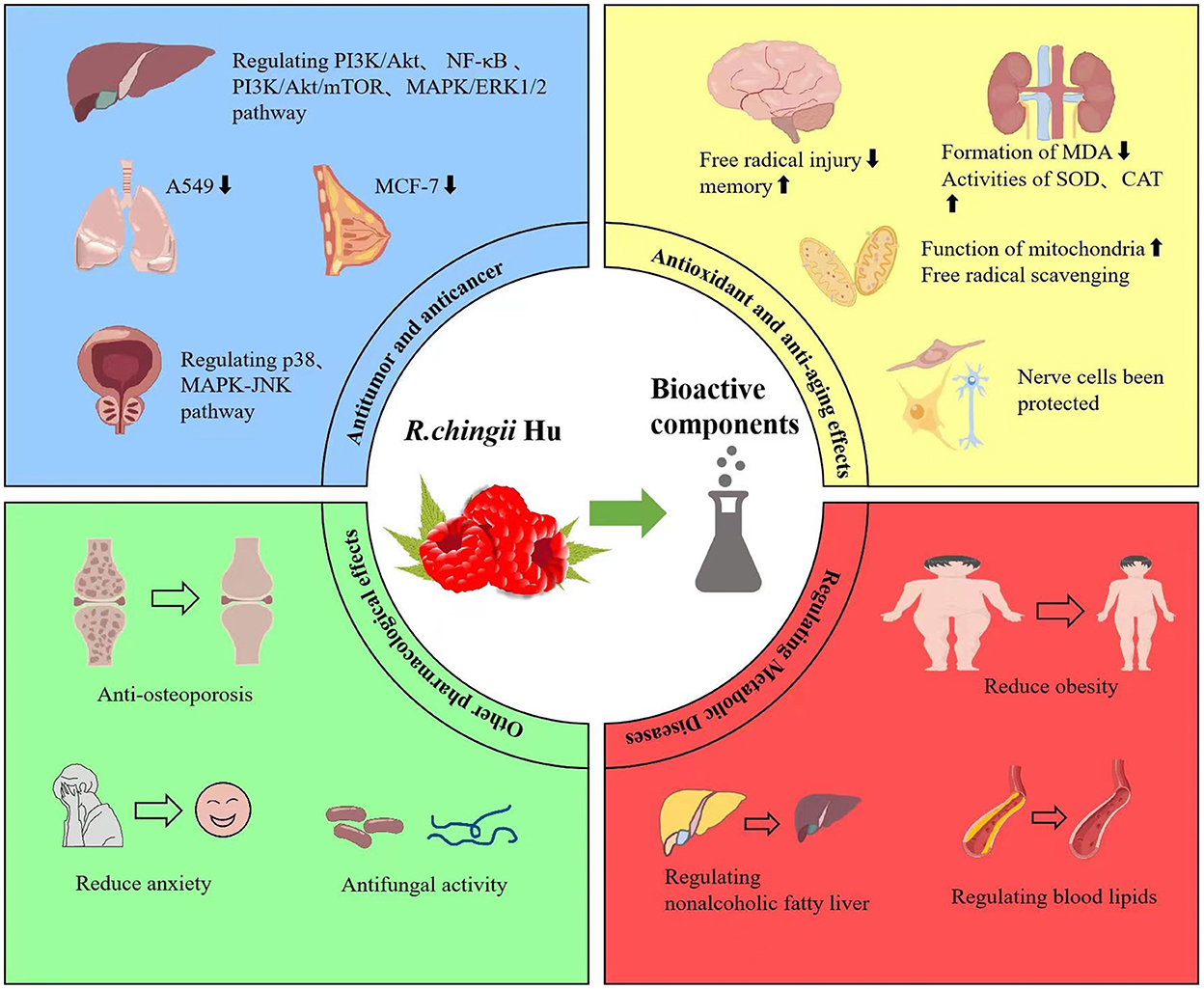
As a famous medicinal plant in TCM, its fruit and leaves are widely used to treat various diseases. Its main pharmacological properties include anti-tumor, anti-aging, anti-coagulant, anti-diabetes, liver-protective, anti-inflammatory, neuroprotective, and anti-osteoporosis activities (Figure 4, Table 2).
Anti-tumor and anti-cancer effects
The anti-tumor and anti-cancer effects of R. chingii Hu and its bioactive components get more and more attention, which are supported by a large number of experiments in vivo and in vitro. It is found that R. chingii Hu extract inhibited the proliferation of hepatoma cell line SMMC-7721, and cisplatin could enhance the effect (44). Zhang et al. (45) investigated anti-cancer activity of four effective components extracted from R.chingii Hu (flavonoids, polysaccharides, saponins, and alkaloids) against human lung adenocarcinoma A549 cells in vitro. They found that flavonoids have the strongest anti-cancer activity among the four chemical components. Zhang et al. (25) also isolated fruit and leaf polysaccharides from R. chingii Hu and compared their anti-inflammatory, anti-oxidant and anti-cancer activities against breast cancer cells MCF-7 and liver cancer cells Bel-7402. They found that the biological activity of leaf polysaccharides is better than that of fruit polysaccharides. They further found that the ethyl acetate fraction of R. chingii extracted with 95% ethanol had the strongest cytotoxicity against human cancer cell lines (HepG-2, Bel-7402, A549, and MCF-7) by active component–tracking approach. Tormentic acid was further isolated from the fraction, which has strong cytotoxicity activities against tumor cell lines as mentioned previously (46).
Ellagic acid belongs to polyphenols. R. chingii Hu is rich in ellagic acid. Raspberry ellagic acid has a good effect in inhibiting several types of tumors or cancers and has anti-mutagenic and anti-cancer effects. Zhong chen confirmed the inhibitory effect of ellagic acid on hepatocellular carcinoma (HCC) (47). It makes the cell cycle of HepG2 stagnate in G0/G1 phase and can further promote the apoptosis of HepG2 cells and induce DNA damage. Cui shanshan found a significant positive correlation between the anti-oxidant activity of raspberry ellagic acid in vitro and the anti-proliferation activity of lung cancer cell A549, which confirmed that raspberry ellagic acid had an obvious inhibitory effect on the proliferation of lung cancer cell A549 (48). Ellagic acid can inhibit endometrial cancer cell proliferation, inhibit the cell cycle, and promote apoptosis via the PI3K signaling pathway in endometrial cancer (49). Urolithin A, a metabolite of the intestinal microbiota-derived from ellagic acid, reaches a high concentration in the human colon and potentiates the anti-cancer effects of 5-fluorouracil chemotherapy on human colon cancer cells (50).
Oleanolic acid (OA) and ursolic acid (UA) are pentacyclic triterpenoids, which are rich in R. chingii Hu. OA and UA are well-known for their anti-cancer activity. OA and UA had an effect on inhibiting NF-KB activation, so as to inhibit the growth of hepatocarcinoma cell lines (HepG2, Hep3B, and HA22T/VGH) (51). Li et al. (52) have shown that OA inhibited cell viability and proliferation in a dose-dependent manner and promoted cell apoptosis and G0/G1 phase cell cycle arrest in prostate cancer cells (PC-3, DU145, and LNCaP). In addition, they have shown that OA exerts anti-cancer effects on PC-3 and DU145 cells in vitro by inhibiting the PI3K/Akt pathway.
β-sitosterol (BS), a phytosterol derived from R. chingii Hu, has anti-cancer properties against breast cancer, prostate cancer, colon cancer, lung cancer, stomach cancer, ovarian cancer, and leukemia (53). Researchers have demonstrated that BS induced apoptosis and suppressed the proliferation of ovarian cancer cells. In addition, researchers have found that BS showed synergistic anti-cancer effects in combination with standard anti-cancer drugs. Due to its low risk of side effects, BS may be a potential anti-cancer drug (54).
Tiliroside, the major representative of the R. chingii Hu flavonoid, can induce the apoptosis of human lung cancer cells A549 and inhibit tumor cell proliferation (45). Tiliroside exerted a significantly higher anti-proliferation effect on liver cancer cell lines Hep3B and SNU-449 and modulates E2Fs/Caspase-3 axis (55).
Quercetin is a flavonoid present in R. chingii Hu, which possesses anti-cancer properties via PI3K/Akt/mTOR, Wnt/β-catenin, and MAPK/ERK1/2 pathways to promote the loss of cell viability, apoptosis, and autophagy (56). Ghafouri-Fard et al. (35) summarized the recent data about the preventive and therapeutic influences of quercetin in prostate cancer and found out that quercetin might prevent the initiation of prostate cancer as it indirectly blocks the activity of promoters of two important genes in the pathogenesis of prostate cancer, i.e., AR and PSA. Other than that, quercetin might enhance the effects of other therapeutic options against prostate cancer.
Hyperoside (HY) inhibited the viability of human non-small cell lung cancer A549 cells in a time- and dose-dependent manner and induced apoptosis via the p38 MAPK- and JNK-induced mitochondrial death pathway (57).
Kaempferol 3-rutinoside, as a quality control index component of R. chingii Hu, the study showed a high anti-tumor activity against colon cancer cell lines (T84 and HCT-15) in anti-proliferative assays via overexpressing of caspases 3, 8, and 9 and activating autophagy (58). Eltamany et al. (59) used molecular docking and revealed that kaempferol-3-rutinoside was one of the most active inhibitors of Bcl-2. Thus, very little is known about the pharmacology of kaempferol 3-rutinoside. Further investigation of the pharmacological effects of kaempferol 3-rutinoside and the underlying mechanisms of anti-tumor property will require additional and more precise in vitro and clinical trials.
As one of the essential roles in R. chingii Hu extract, alkaloids have shown anti-tumor effects in various studies. In recent decades, it is clearly confirmed that diverse alkaloid classes induced apoptosis in both syngeneic and xenograft tumors (6, 25, 57). In addition to the induction of apoptosis, alkaloids might cause autophagy in tumors, it can occur either alone or together with apoptosis upon treatment with some plant alkaloids. It may also amputate the supply of nutrients and oxygen from the surrounding normal tissue environment by inhibiting tumor angiogenesis. The underlying mechanisms were revealed, such as the downregulation of VEGF and its receptors, as well as signaling molecules such as AKT, SRC, FAK, ERK, p53, and transcription factors such as NF-κB and mTOR. Activating or shutting off multiple signal pathways by affecting transcription factors including NF-κB, MYC, FOS, CREB, WT1, and mTOR may also be the reason for its anti-tumor effects. Researchers have also found the inhibition of metastases by alkaloids in vivo or inhibition of primary tumors in vivo. In conclusion, alkaloids may play an important role in the treatment of tumor, its anti-tumor effects are well established, but whether its toxic effects are dose-related remains to be studied.
Anti-oxidant, improving learning and memory ability, and anti-aging effects
Traditional Chinese medicine believes that Fu-Pen-Zi is a good medicine for tonifying the kidney and anti-aging. Modern researchers believe that Fu-Pen-Zi has anti-oxidant, improving learning and memory ability and anti-aging effects (6). Studies have shown that polysaccharides and glycoproteins of R. chingii Hu have obvious anti-oxidant effects and can effectively scavenge free radicals (26, 60, 61). A study found that a novel glycoprotein was isolated and purified from Fu-pen-zi (R. chingii Hu.) could significantly inhibit the formation of malondialdehyde (MDA) and raise the activities of superoxide dismutase (SOD) and catalase (CAT) in mice kidney and serum. It is suggested that the anti-aging mechanism of R. chingii Hu may be realized by increasing Klotho gene expression and repairing renal function (26, 60, 61). Chen et al. (61) found that the anti-oxidant activities of the three parts of R. chingii Hu followed the decreasing order of young leaves>old leaves and stems>fruits. It is a potential source of natural phenolic anti-oxidants and is promising for the development of healthy foods. The Fu-Pen-Zi extract can improve the learning and memory ability of natural aging rats, especially improving the brain cholinergic function and reducing the brain free radical injury of aged rats (62). The high-dose group of raspberry water extract had the best effect on the memory impairment model caused by scopolamine and sodium nitrite, while the high-dose group of raspberry had the best effect on the memory impairment mouse model caused by 40% ethanol (63). Raspberry extract promotes the longevity and stress tolerance of Caenorhabditis elegans through insulin/IGF signaling pathway and DAF-16 (63). The biomolecules in raspberry fruit can be decomposed into urolithin A (UA) in human intestine, which can fight against aging by improving the function of mitochondria (64).
Tiliroside, the main quality marker of R. chingii Hu, has attracted widespread attention for its wide range of bioactive effects including its anti-oxidant and anti-aging activities. Investigations conducted by Corrêa et al. (65) on anti-oxidant property of tiliroside confirmed the anti-oxidant activity in vivo tests, it may have therapeutic potential against oxidative stress–related disorders. Additionally, Li et al. (66) showed that tiliroside performed more effectively as a cytoprotective and anti-oxidant agent than astragalin. Parallel to the aforementioned studies, Velagapudi et al. (67) had also found in an in vitro study that tiliroside inhibited neuroinflammation in BV2 microglia through a mechanism involving TRAF-6 mediated activation of NF-κB and p38 MAPK signaling pathways and concluded that these activities are possibly due to the anti-oxidant property of tiliroside. Tiliroside also activated the proteasome in normal human fibroblasts and delayed cellular senescence, suggesting that it may also affect the aging rate of human cells (68).
Ellagic acid (EA) belongs to phenolic acid, which was found in numerous fruits and vegetables, particularly in R. chingii Hu. It is a natural anti-oxidant that has been attributed to its free radical scavenging activity. EA shows a positive correlation with a low incidence of chronic diseases, especially ulcerative colitis, Cronh's disease, Alzheimer's disease, and diabetes (69). EA (50 mg/kg body weight) treatment can significantly reduce serum liver enzyme activity and reduce serum levels of total bilirubin and direct bilirubin, which may improve its anti-oxidant capacity and alleviate severe liver injury caused by excessive production of free radicals (70). In addition, EA exerts its anti-oxidant effect by activation of the nuclear erythroid 2-related factor 2 (Nrf2), while Nrf2 knockdown diminished the antioxidant effect of EA (71). In an animal model of aging, daily and oral administration of EA exerted an anti-aging effect associated with peroxisome proliferator-activated receptor-γ (PPAR-γ) (72).
Quercetin is also the main characteristic bioactive component extracted from R. chingii Hu. Quercetin has been proven to be an excellent anti-oxidant in vitro because there are two anti-oxidant pharmacophores with the best free radical scavenging configuration in the molecule (72). Applying trolox equivalent anti-oxidant capacity (TEAC) assay to assess the total anti-oxidant capacity, Arts et al. (73) identified that the contribution of quercetin to the total plasma anti-oxidant capacity was 6.24 times greater than that of trolox, the reference anti-oxidant, and concluded that quercetin has the ability to greatly enhance the protection of endogenous anti-oxidants.
Kaempferol 3-rutinoside, the quality marker of R. Chingii Hu, showed protection against kidney damage involved in apoptosis, necrosis, inflammation, and oxidative stress (74). Based on the data from in vivo studies, Sun et al. demonstrated that kaempferol 3-rutinoside along with quercetin-3-rutinoside, quercetin-3-glucoside, and kaempferol-3-glucoside metabolized and absorbed in the form of glycosides for hepatic metabolism in the oxidative stress model showed abilities to increase the total anti-oxidant capacity (T-AOC), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) activity, and significantly increase glutathione (GSH) content, while malondialdehyde (MDA) content was decreased. These results indicated that kaempferol 3-rutinoside can effectively prevent some chronic diseases caused by oxidative stress. It also provides a basis for the effectiveness of R. Chingii Hu as traditional functional food (75). Studies on the bioactive effects of kaempferol 3-rutinoside have not been carried out separately, and the current studies on kaempferol 3-rutinoside have been conducted together with other active substances, so the anti-oxidant activity of kaempferol 3-rutinoside still need more accurate experimental studies.
Ursolic acid (UA), one of the main compounds of R. Chingii Hu, causes increasing attention as a natural anti-oxidant. The IC50 values of UA against DPPH and SOD were 1,721 ± 30.6 and 392 ± 53.57 μg/ml, respectively. The binding affinity of UA inhibiting SOD enzyme was −5.4 kcal/mol, which was higher than that of quercetin as a positive control. The anti-oxidant activity of UA is related to the inhibition of SOD mutation (76). An ex vivo study on the modulatory effect of ursolic acid on neurodegenerative activities in oxidative brain injury showed that UA may protect against the neurological degeneration caused by oxidative injury of FeSO4 in isolated rat brains (77).
All the aforementioned studies showed that the extracts of R. chingii Hu and its bioactive components have strong anti-oxidant activity, and whether there is a synergistic or antagonistic effect between these substances is not known and needs further experimental evidence.
Regulating metabolic diseases (obesity, T2DM, and NAFLD)
Obesity has become a worldwide epidemic issue that poses substantial health problems for both individuals and society. It is well established that obesity brings with it a range of complications, including T2DM and NAFLD (78). It has been proved that R. Chingii Hu extract and its active components have good effects in reducing blood lipids, improving obesity, reducing blood sugar, and improving lipid metabolism (76, 79, 80). Fan et al. studied the hypolipidemic effect of R. Chingii Hu leaf on hyperlipidemia model animals and hyperlipidemia adults. The experimental adult group was given R.chingii Hu leaf drinking in the dose of three times/day and 2 g per time without changing daily custom and diet for 30 days. The experimental animal group was given 0.5, 1.0, and 2.0 g/kg bw for 30 days. In animal experiments, serum TC and TG in R.chingii Hu leaf 1.0 and 2.0 g/kg bw groups were significantly lower than those in high-fat model group (P < 0.05). In the adult trial, serum TC and TG were significantly lower than the control group (P < 0.05). The total effective rate of reducing blood lipids in the experimental group was 80.4%. R. chingii Hu leaves show significant effects of decreasing serum lipid levels in rats and human adults as well (79). The methanolic extract of R. chingii fruits exhibited significant protein tyrosine phosphatase 1B (PTP1B) inhibitory activity. The inhibitor of PTP1B, which has the potential to anti-diabetes, anti-obesity, and anti-cancer drugs, has attracted the interest of researchers (79). Polysaccharide from R. chingii Hu provides protection against palmitic acid–induced lipotoxicity in human hepatocyte cell line LO2, which can reduce oxidative stress by preventing the accumulation of reactive oxygen species (ROS), reducing the collapse of mitochondrial membrane potential (MMP), and reducing the decrease of glutathione (GSH) (81).
Ellagic acid (EA) seems to play an anti-diabetic activity through the action on β-cells of the pancreas, stimulating insulin secretion and decreasing glucose intolerance (82). EA promoted the browning of white adipose tissue (WAT) in obese rats by inhibiting the white adipocyte maintaining gene and improved obesity-induced dyslipidemia and hepatic steatosis. In particular, EA improved the expression of a specific protein of the brown adipocyte UCP1 gene in WAT (83).
Studies in cells and rodents have suggested an important role for Raspberry ketone (RK) in hepatic/cardio/gastric protection and as an anti-hyperlipidemic, anti-obesity via mediating activation of PPAR-α (84). RK has been used as an over-the-counter weight loss product, but it is still controversial, and the mechanism is still unknown.
Raspberry polyphenols have beneficial effects in regulating hepatic lipid metabolism and inflammation in rats with nonalcoholic fatty liver caused by an obesogenic diet (85). Pelargonidin-3-O-glucoside derived from raspberry exerts plays a role in regulating blood glucose by inducing autophagy and modulating gut microbiota (86).
Oleanolic acid (OA) and ursolic acid (UA) are the main characters extracted from R. Chingii Hu. In addition to the biological activities mentioned previously, it also exhibits strong inhibitory activity on α-glucosidase. Kalaycioglu et al. (87) detected the relationship between oleanolic acid and ursolic acid and α-glucosidase, and the inhibitory effect inferred that oleanolic acid and ursolic acid have great potential in the management of diabetes. UA and its synthetic derivatives demonstrated excellent anti-diabetic, anti-obesity, anti-hyperlipidemic, and anti-cardiovascular properties (88). This provides the basis for developing UA into a therapeutic agent for the prevention or treatment of metabolic diseases.
Other pharmacological effects
The ethanol extract of R. chingii leaves can play an effective anti-thrombotic role in vivo and in vitro. Raspberry A and raspberry B in R. chingii can prevent osteoclast activity and bone absorption, while quercetin and kaempferol can stimulate osteoblast activity and further play an anti-osteoporosis role (36). The extract from R. chingii can attenuate anxiety-like behavior induced by ethanol withdrawal by modulating NE in sthe hippocampus in rats (89). The ultrasonic extraction from R. Chingii Hu fruit has good anti-fungal activity against a variety of pathogenic fungi, and the anti-fungal constituents may come from its triterpenoid components (90). The raspberry extract has a removing chloasma effect, which can decrease chloasma color and area (91). The extract of R. chingii Hu has the function of nourishing the kidney and strengthening the Yang-qi effect and treating male infertility and impotence (6, 92). R. chingii Hu polysaccharides are mainly through TLR2-dependent MAPK and NF-κB and jak-STAT pathways that regulate the immune response of macrophages (93).
Toxicity
Although R. chingii Hu has long been considered a safe dual-use food-medicine herb, due to its wide consumption, it is of great significance to determine whether chronic intake will produce toxicological effects. The safety of R. chingii Hu should also be given more attention. We summarize as follows. Ji et al. (94) have shown that R. chingii Hu extract had no toxic effect on HepG2 cells at 5–160 mg.L−1 mass concentration via MTT method. R. chingii Hu extract has an obvious protective effect on acute liver injury induced by ConA in mice. Tang et al. (95) have found that the maximum tolerated oral dose of R. chingii Hu was more than 20.0 g/kg body weight in mice via acute toxicity test. According to the acute toxicity classification standard, Hubei R. chingii Hu is non-toxic. No mutagenicity was found by bone marrow cell micronucleus test, Ames test, and sperm abnormality test in Kunming mice. In the sub-chronic toxicity study, after 90 days of intragastric administration of R. chingii Hu leaf extract at doses of 2.5, 5.0, and 10.0 g/(kg.d) to Wistar rats, no death or clinical poisoning symptoms were found, and there were no abnormal changes in hematology, biochemistry, and histopathology. These results have shown that Hubei R. Chingii Hu can be safely used as a source of healthy food and medicine. However, the current research on safety and toxicology is relatively limited. Therefore, before pharmacological test and clinical application, it is necessary to verify the safety of R. chingii Hu.
Quality control
In recent years, with the widespread use of R. chingii Hu medicinal materials and their preparations, R. chingii Hu's quality control evaluation has received great attention. From qualitative analysis to quantitative analysis, from single index to multiple index development, the new TCM quality control mode is of great practical significance for controlling the quality of TCM, especially to ensure the clinical safety and effectiveness of TCM. In the 2015 edition of Chinese Pharmacopoeia (Chinese Pharmacopoeia Commission, 2015), the content of R. chingii Hu was determined by HPLC with ellagic acid and kaempferol-3-o-rutoside as the index components. Furthermore, the contents of ellagic acid and kaempferol-3-rutinoside in the fruits of R. chingii should not be less than 0.20 and 0.03%, respectively. Zeng et al. (96) established the HPLC fingerprint of R. chingii Hu from different producing areas and the content determination method of total flavonoids. Sun et al. (97) use ellagic acid and five flavonoid ingredients (rutin, hypericin, isquercetin, kaempferol-3-O-rassoside, and linoside) as the index components and determined the contents of 17 batches of different R. chingii Hu by HPLC. Ma et al. (98) determined the contents of four flavonoids by HPLC with astragaloside, Bodhi glycoside, quercetin, and kaempferol as index components. Xu et al. (99) prepared 12 batches of R. chingii Hu standard decoction, the content of ellagic acid was determined, and the transfer rate was calculated. The extraction rate was determined, and the HPLC fingerprint analysis method of R. chingii Hu standard decoction was established. Xu et al. (99) established an HPLC method for the determination of tiliroside and kaempferol in R. chingii Hu. Ceci et al. (100) and Ping et al. (30) conducted combined transcriptomic and metabolic analyses of R. chingii fruits from different developmental stages and revealed the mechanisms of the fruit development and quality control of R. chingii.
Developing quality control methods, among them, the research on pharmacodynamic components is the premise and cornerstone to support the quality control of traditional Chinese medicines. The research on biological potency methods can well reflect the overall activity and efficacy of traditional Chinese medicine products and play a role in the safety and effectiveness of product quality–related clinical applications. The quality assurance of R. chingii Hu has important practical significance for the clinical safety and effectiveness of traditional Chinese medicine. We believe that the quality control of R. chingii Hu should be characterized by the local pharmacopoeia standards (101), with ellagic acid and kaempferol-3-o-rutoside as characteristic indicator components.
Drug development and utilization status
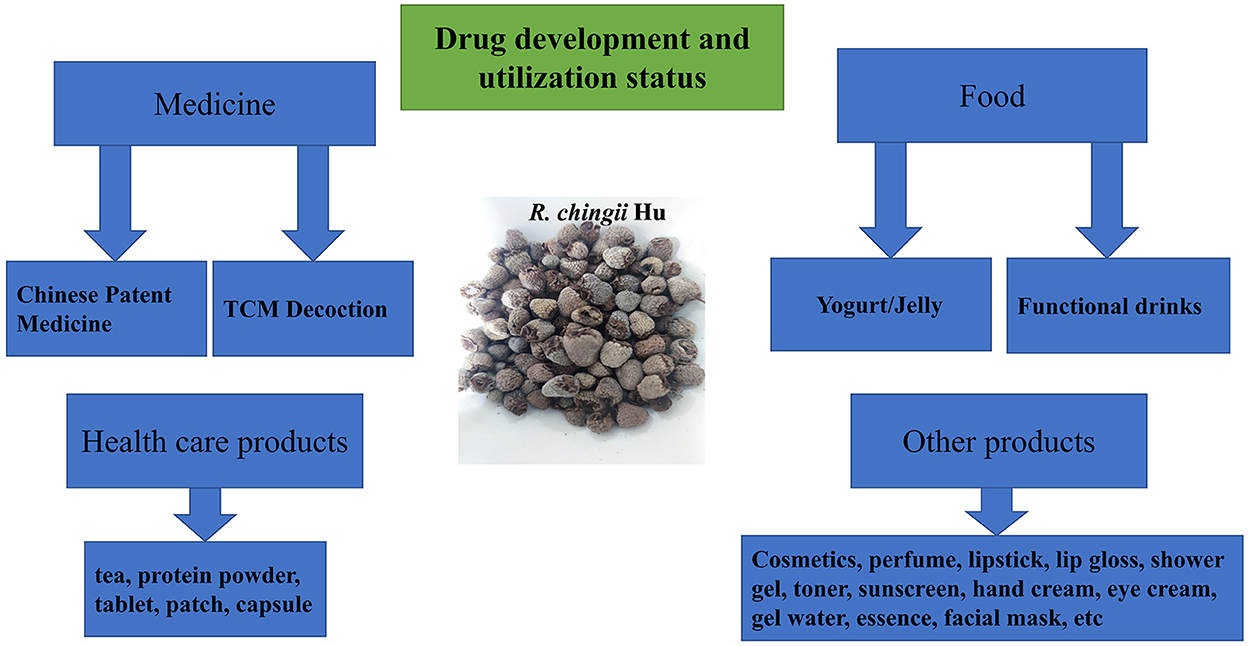
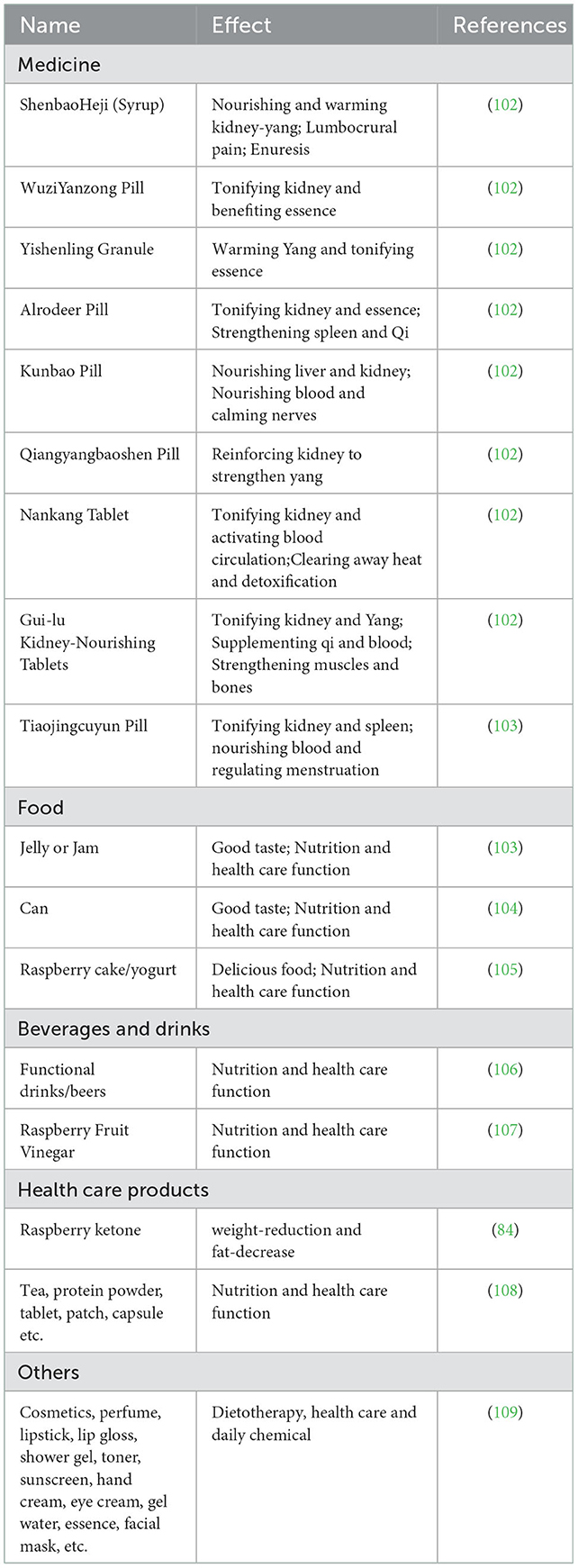
The research and development of R. chingii Hu–related drugs, health care products, and daily chemical products have attracted more attention for scholars. The number of documents and patent applications about R. chingii Hu is increasing rapidly. As a traditional Chinese medicine, R. chingii Hu has been widely used in kidney deficiency enuresis, frequent urination, impotence, premature ejaculation, spermatorrhea, and other diseases. In 2019, the planting area of R. chingii Hu is 8586.7 hm2, an increase of 5.8% compared with the same period of the previous year. At present, the drugs containing R. chingii Hu sold on the market and included in the Chinese Pharmacopoeia mainly include WuziYanzong pill, ShenbaoHeji, and Yishenling granule. WuziYanzong Pill is composed of Lyciumbarbarum, dodder seed, R. chingii Hu, Schisandra chinensis, and plantaginis semen. R. chingii Hu plays the role of tonifying kidney and benefiting essence. R. chingii Hu, the official medicine of ShenbaoHeji, is similar to S.chinensis. Together, they play the role of strengthening the kidney, stopping bleeding, and astringent essence. Therefore, in the direction of drug development of R.chingii Hu, we should give priority to the important role of R. chingii Hu in tonifying the kidney. In addition, R. chingii Hu also has a variety of pharmacological effects, including eyesight, weight loss, hypoglycemic, and anti-aging. As a new nutritional supplement, raspberry ketone is mainly used to treat or prevent obesity or obesity-related diseases. Overall, R. chingii Hu–related drug development still has broad prospects.
Due to the homologous characteristics of R. chingii Hu medicine and food, it is made into health care products with health care efficacy together with other excipients, such as R. chingii Hu tea, capsule, and buccal tablet with health care efficacy. There are more than 50 kinds of domestic health food containing R. chingii Hu on the market. By compounding R. chingii Hu with different excipients, a series of health products with different effects are made. Jiangxi Tianhai Technology Co., Ltd. made an R. chingii Hu tablet with anti-aging and improving immunity effect. R. chingii Hu–related daily chemical products include cosmetics, perfume, lipstick, lip gloss, shower gel, toner, sunscreen, hand cream, eye cream, gel water, essence, and facial mask.
Chinese Wild R. chingii Hu is rich in resources, widely distributed and various, but it has not been well developed and utilized, and the research and development of products are mostly concentrated. Relying on the resource advantages of R. chingii Hu in China, the research on active components and functional factors of R. chingii Hu was carried out in depth. Building a product system based on the needs of different groups, designing and developing healthy products with clear functional factors and precise efficacy positioning will help to open up the domestic and foreign markets of R. chingii Hu products. The drug development and utilization status of R. chingii Hu are shown in Figure 5, Table 3.
Conclusions and future perspectives
In conclusion, R. chingii Hu is a very valuable food-medicine herb. The therapeutic application of R. chingii Hu in a variety of diseases has shown potential for improving human health. In this review, we summarized several studies showing that the main bioactive components of R. Chingii Hu, including terpenoids, flavonoids, steroids, and alkaloids, may exert their anti-inflammation, anti-oxidation, radical scavenging, and anti-angiogenesis effects to be a cardioprotectant, lower the cancer risk, control the obesity, and alleviate diabetes. However, all those studies focused on the function of one of the components of diseases, it is not clear as yet how or whether these complex polyphenolic compounds have synergy effects or antagonism under certain conditions. The data on clinical application remain a major weakness in this area. When it comes to its clinical application, there are still many questions to be answered, how to solve the lower bioavailability and solubility particularly. There is an urgent need to carry out long-term randomized controlled trials in relevant populations to promote the clinical application of R. chingii Hu.
We reviewed the R. chingii Hu toxicity and quality control. There are relatively few studies on R. chingii Hu toxicity, but in general, it is safe food and medicine, and more research needs further research. By analyzing the pharmacological effects of R. chingii Hu and its specific chemical components of it, the quantity markers can be ellagic acid, kaempferol-3-o-rutoside, quercetin, and tiliroside. With the development of science and technology, the research on the quality control of TCM becomes more and more comprehensive. Safety and effectiveness are the foundation of medicine, and the quality evaluation of R. chingii Hu should link with pharmacological efficacy and safety.
The development of R. chingii Hu–related drugs is relatively single, which is limited to TCM and prescriptions. The development and utilization of its effective components has great development prospects. At the same time, with the development of large-scale health industry, therapeutic products have gradually become a new consumption trend. Although R. chingii Hu has been developed into therapeutic and health products, its large-scale development and utilization are limited, resulting in the output unable to meet the needs of the market. Wild R. chingii Hu resources in China are widely distributed, rich in resources and various types, but they have not been well developed and utilized. Therefore, it is necessary to pay attention to R. chingii Hu and its bioactive components in the future and expand its clinical application.
Author contributions
BH: writing—original draft. LD, LJ, and ML: writing—editing. YL: visualization. XL and ZW: editing. GK: writing—review, editing, and supervision. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Major Science and Technology Projects of Breeding New Varieties of Agriculture in Zhejiang Province (2021C02074-3), the National Natural Science Foundation of China (81522049, 82073963, and 31571735), Natural Science Foundation of Zhejiang Province of China (LY19H270002 and LGF22H290001), the Zhejiang Provincial Ten Thousands Program for Leading Talents of Science and Technology Innovation (2018R52050), the Zhejiang Provincial Program for the Cultivation of High level Innovative Health Talents, Research Project of Zhejiang Chinese Medical University Research Foundation (2021JKZDZC06). We appreciate the experimental support from the Public Platform of Medical Research Center, Academy of Chinese Medical Science, Zhejiang Chinese Medical University.
Acknowledgments
We acknowledge Maoxiang Yan and Fang Wang for providing support and assistance for this article.
Conflict of interest
ZW was employed by Zhejiang Research Institute of Traditional Chinese Medicine Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
Akt, Protein Kinase B; BS, β-sitosterol; CAT, catalase; CNKI, China national knowledge infrastructure; CREB, cAMP-response element binding protein; CVD, cardiovascular disease; DPPH, 1,1-diphenyl-2-picrylhydrazyl; EA, Ellagic acid; ERK, xtracellularsignal-regulatedkinase; FAO, Food and Agriculture Organization; GSH, glutathione; GSH-Px, glutathione peroxidase; HCC, hepatocellular carcinoma; HPLC, High Performance Liquid Chromatography; HT, Hydrolyzable Tannin; HY, Hyperoside; IGF, insulin like growth factor; jak-STAT, Janus tyrosine Kinase-Signal Transducer and Activator of Transcription; MAPK, mitogen-activated protein kinase; MDA, malondialdehyde; MMP, mitochondrial membrane potential; mTOR, Mechanistic Target Of Rapamycin; NAFLD, nonalcoholic fatty liver disease; NF-κB, nuclear factor-kappa B; Nrf2, nuclear erythroid 2-related factor 2; OA, Oleanolic acid; PI3K, Phosphatidylinositol-3-kinase; PPAR, peroxisome proliferator-activated receptor; PTP1B, protein tyrosine phosphatase 1BRK, Raspberry ketone; ROS, reactive oxygen species; SOD, superoxide dismutase; T2DM, type 2 diabetes mellitus; T-AOC, total anti-oxidant capacity; TC, total cholesterol; TCM, traditional Chinese medicine; TEAC, trolox equivalent anti-oxidant capacity; TG, triglyceride; TLR2, Toll-Like Receptor 2; UA, ursolic acid; UCP1, uncoupling protein 1; VEGF, vascular endothelial growth factor; WAT, white adipose tissue; WHO, World Health Organization; WT1, Wilms Tumor 1 protein.
References
1. Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. (2018) 20:12. doi: 10.1007/s11906-018-0812-z
2. Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic steatohepatitis: a review. Jama-J Am Med Assoc. (2020) 323:1175–83. doi: 10.1001/jama.2020.2298
3. Li Z, Zhang Z, Ren Y, Wang Y, Fang J, Yue H, et al. Aging and age-related diseases: from mechanisms to therapeutic strategies. Biogerontology. (2021) 22:165–87. doi: 10.1007/s10522-021-09910-5
5. Que L, Yang G, Li Y, Shan F, Huang LQ. Overview of revision of the catalogue of the substances traditionally considered as both food and chinese medicine. Chin Pharmaceut J. (2017) 52:521–24. doi: 10.11669/cpj.2017.07.001
6. Yu G, Luo Z, Wang W, Li Y, Zhou Y, Shi Y. Rubus chingii hu: a review of the phytochemistry and pharmacology. Front Pharmacol. (2019) 10:799. doi: 10.3389/fphar.2019.00799
7. Bai X. Summary of Revised Contents of Medical Standards of Preparation Recorded in Chinese Pharmacopeia. Beijing: Chinese Pharmacopoeia Commission (2011).
8. Chinese Pharmacopoeia Commission, 2015. Pharmacopoeia of People's Republicof China. Part I. Beijing: People's Medical Publishing House (2015).
9. He BW, Jiang JP, Xu DB. Genuine medicinal materials “Zhe-ba-wei” and new “Zhe-ba-wei”. New Countryside. (2020) 05:21–22.
10. Liu MX, Niu JE. Advance research on raspberry (Rubus chingii Hu) and resource utilization. Sci Technol Vis. (2014) 22:26–7. doi: 10.3969/j.issn.2095-2457.2014.22.015
11. Sheng JY, Wang SQ, Liu KH, Zhu B, Zhang QY, Qin LP, et al. Rubus chingii Hu: an overview of botany, traditional uses, phytochemistry, and pharmacology. Chin J Nat Med. (2020) 18:401–16. doi: 10.1016/S1875-5364(20)30048-0
12. Sun N, Wang Y, Liu Y, Guo ML, Yin J. A new ent-labdane diterpene saponin from the fruits of Rubus chingii. Chem Natural Compounds. (2013) 49:49–53. doi: 10.1007/s10600-013-0503-6
14. Tanaka T, Kohda H, Tanaka O, Chen FH, Leu JL. Rubusoside (β-D-Glucosyl ester of 13-O-β-D-glucosyl-steviol), a sweet principle of Rubus chingii Hu (Rosaceae). J Agric Chem Soc Jpn. (1981) 45:2165–66. doi: 10.1271/bbb1961.45.2165
15. Zheng Q, Wu L, Zhang K. Reseaech overview and product development trend analysis of raspberry. J Chin Med Mater. (2019) 42:1204–8. doi: 10.16178/j.issn.0528-9017.20210124
16. Shi Y. Raspberry research progress of nutritional ingredients and pharmacological effects. Shandong Chem Ind. (2017) 46:71–2.
17. Kuilin C, Darong H, Shaojie H, Jing Y, Huiying Z, Bing D. Research progress on active components of raspberry and their comprehensive utilization. Food Mach. (2022) 38:219–26. doi: 10.13652/j.s.PJX.1003.5788.2022.90216
18. Kejun C. I. Study on Active Components of Raspberry II. Study on the Stability of Stilbenes in Gorse Root. Fudan: Fudan University (2008).
19. Ding HY. Extracts and constituents of Rubus chingii with 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Free Radical Scavenging Activity. Int J Mol Sci. (2011) 12:3941–49. doi: 10.3390/ijms12063941
20. Cui L, Zheng ZQ. About the study of progress on the chemical composition and pharmacological effects of Rubus chingii Hu. Electron J Gen Stomatol. (2020) 7:192–96.
21. Guo QL, Yang JS, Liu JX. Studies on chemical constituents in fruits of Rubus chingii. Chin Pharmaceut J. (2007).
22. Chen Z, Jiang J, Shu L, Li X, Huang J, Qian B, et al. Combined transcriptomic and metabolic analyses reveal potential mechanism for fruit development and quality control of Chinese raspberry (Rubus chingii Hu). Plant Cell Rep. (2021) 40:1923–46. doi: 10.1007/s00299-021-02758-6
23. Desai AJ, Dong M, Miller LJ. Beneficial effects of β-sitosterol on type 1 cholecystokinin receptor dysfunction induced by elevated membrane cholesterol. Clin Nutr. (2016) 35:1374–9. doi: 10.1016/j.clnu.2016.03.003
24. Wang L, Lei T, Han G, Yue J, Zhang X, Yang Q, et al. The chromosome-scale reference genome of Rubus chingii Hu provides insight into the biosynthetic pathway of hydrolyzable tannins. Plant J. (2021) 107:1466–77. doi: 10.1111/tpj.15394
25. Zhang T, Lu C, Jiang J, Wang M, Wang D, Zhu W. Bioactivities and extraction optimization of crude polysaccharides from the fruits and leaves of Rubus chingii Hu. Carbohydrate Polymers. (2015) 130:307–15. doi: 10.1016/j.carbpol.2015.05.012
26. Huihui K, Tao B, Wei C. New function of polysaccharide from Rubus chingii Hu: protective effect against ethyl carbamate induced cytotoxicity. J Sci Food Agric. (2021) 101:3156–64. doi: 10.1002/jsfa.10944
27. Huixia Z, Jinxu S, Hao S. Analysis of raspberry polysaccharide monosaccharide components by HPLC. Shandong Chem. (2019) 48:108–10.
28. Lu Y. Extraction and Fraction of Hypoglycemic Components From Rubuschingii Hu and Identification Of major Bioactive Compound. Jiangxi: Jiangxi Normal University (2020).
29. Dian LH, Gong XL, Cai C, Zhang LJ. Analysis of volatile oils in Rubus chingii Hu by GC-MS. Tianjin Pharm. (2005) 14:9–10.
30. Ceci C, Lacal P, Tentori L, Martino M, Graziani G. Experimental Evidence of the antitumor, antimetastatic and antiangiogenic activity of ellagic acid. Nutrients. (2018) 10:1756. doi: 10.3390/nu10111756
31. Diao M, Liang Y, Zhao J, Zhang J, Zhang T. Complexation of ellagic acid with α-lactalbumin and its antioxidant property. Food Chem. (2022) 372: 131307. doi: 10.1016/j.foodchem.2021.131307
32. Castellano JM, Ramos-Romero S, Perona JS. Oleanolic acid: extraction, characterization and biological activity. Nutrients. (2022) 14:623. doi: 10.3390/nu14030623
33. Sun Q, He M, Zhang M, Zeng S, Xu H. Ursolic acid: a systematic review of its pharmacology, toxicity and rethink on its pharmacokinetics based on PK-PD model. Fitoterapia. (2020) 147:104735. doi: 10.1016/j.fitote.2020.104735
34. Hussain H, Green IR, Ali I, Khan IA, Ali Z, Al-Sadi AM, et al. Ursolic acid derivatives for pharmaceutical use: a patent review (2012-2016). Expert Opin Therapeutic Patents. (2017) 27:1061–72. doi: 10.1080/13543776.2017.1344219
35. Ghafouri-Fard S, Shabestari FA, Vaezi S, Abak A, Shoorei H, Karimi A, et al. Emerging impact of quercetin in the treatment of prostate cancer - ScienceDirect. Biomed Pharmacother. (2021) 138:11548. doi: 10.1016/j.biopha.2021.111548
36. Gong J, Liang W, Hospital SP. Chemical and antiosteoporotic activity components from Rubus chingii Hu. Chin Arch Tradit Chin Med. (2016).
37. Cazarolli LH, Zanatta L, Alberton EH, Folador P, Damazio RG, Pizzolatti MG. Flavonoids: prospective drug candidates. Mini Rev Med Chem. (2008) 8:1429–40. doi: 10.2174/138955708786369564
38. Grochowski DM, Marcello L, Sebastian G, Francesco C, Micha? T. A review on the dietary flavonoid tiliroside. Comprehensive Rev Food Sci Food Safety. (2018) 17:1395–421. doi: 10.1111/1541-4337.12389
39. Hua F, Zhou P, Liu PP, Bao G. Rat plasma protein binding of kaempferol-3-O-rutinoside from Lu'an GuaPian tea and its anti-inflammatory mechanism for cardiovascular protection. J Food Biochem. (2021) 45:e13749. doi: 10.1111/jfbc.13749
40. Liz R, Zanatta L, dos Reis GO, Horst H, Pizzolatti MG, Silva FRMB, et al. Acute effect of β-sitosterol on calcium uptake mediates anti-inflammatory effect in murine activated neutrophils. J Pharm Pharmacol. (2013) 65:115–22. doi: 10.1111/j.2042-7158.2012.01568.x
41. Leverton RM. Hypocholesteremic effect of sitosterol. Nutr Rev. (2010) 11:326–28. doi: 10.1111/j.1753-4887.1964.tb04830.x
42. Efferth T, Oesch F. Repurposing of plant alkaloids for cancer therapy: Pharmacology and toxicology. Seminars Cancer Biol. (2019) 68:143–63. doi: 10.1016/j.semcancer.2019.12.010
43. Mittal R, Jaitak V. Plant derived natural alkaloids as new antimicrobial and adjuvant agents in existing antimicrobial therapy. Curr Drug Targets. (2019) 20:1409–33. doi: 10.2174/1389450120666190618124224
44. Hu YL. The research of the inhibition on hepatoma cell line SMMC-7721 by raspberry extract. Shandong Univer Tradit Chin Med. (2015).
45. Zhang TT, Yang L, Jiang JG. Bioactive comparison of main components from unripe fruits of Rubus chingii. Hu and identification of the effective component. Food Funct. (2015) 6:2205–14. doi: 10.1039/C5FO00406C
46. Zhang T-T, Liu Y-J, Yang L, Jiang J-G, Zhao J-W, Zhu W. Extraction of antioxidant and antiproliferative ingredients from fruits of Rubus chingii Hu by active tracking guidance. Medchemcomm. (2017) 8:1673–80. doi: 10.1039/C7MD00240H
47. Zhong C. The Mechanisms of Ellagic acid Against Hepatocelluar Carcinoma and Synergistic Effects of its Combinations with Two Chemotherpaeutic Agents. Harbin: Northeast Forestry University (2019).
48. Cui SS. Preparation of Red Raspberry Ellagic Acid and Its anti-lung Cancer Cells A549 Activity. Jinzhou: Jinzhou Medical University (2020).
49. Wang Y, Ren F, Li B, Song Z, Chen P, Ouyang L. Ellagic acid exerts antitumor effects via the PI3K signaling pathway in endometrial cancer. J Cancer. (2019) 10:29738. doi: 10.7150/jca.29738
50. Gonzalez-Sarrias A, Tome-Carneiro J, Bellesia A, Tomas-Barberan FA, Espin JC. The ellagic acid-derived gut microbiota metabolite, urolithin A, potentiates the anticancer effects of 5-fluorouracil chemotherapy on human colon cancer cells. Food Funct. (2015) 6:1460–9. doi: 10.1039/C5FO00120J
51. Fontana G, Bruno M, Notarbartolo M, Labbozzetta M, Rosselli S. Cytotoxicity of oleanolic and ursolic acid derivatives toward hepatocellular carcinoma and evaluation of NF-κB involvement. Bioorganic Chem. (2019) 90:103054. doi: 10.1016/j.bioorg.2019.103054
52. Li X, Song Y, Zhang P, Zhu H, Chen L, Xiao Y, et al. Oleanolic acid inhibits cell survival and proliferation of prostate cancer cells in vitro and in vivo through the PI3K/Akt pathway. Tumor Biol. (2016) 37:7599–613. doi: 10.1007/s13277-015-4655-9
53. Sayeed MB, Ameen SS. Beta-sitosterol: a promising but orphan nutraceutical to fight against cancer. Nutrit Cancer. (2015) 67:1–7. doi: 10.1080/01635581.2015.1087042
54. Bae H, Park S, Ham J, Song J, Hong T, Choi JH, et al. ER-mitochondria calcium flux by beta-sitosterol promotes cell death in ovarian cancer. Antioxidants (Basel). (2021) 10:1583. doi: 10.3390/antiox10101583
55. Han R, Yang H, Lu L, Lin L. Tiliroside as a CAXII inhibitor suppresses liver cancer development and modulates E2Fs/Caspase-3 axis. Sci Rep. (2021) 11:8626. doi: 10.1038/s41598-021-88133-7
56. Reyes-Farias M, Carrasco-Pozo C. The anti-cancer effect of quercetin: molecular implications in cancer metabolism. Int J Mol Sci. (2019) 20:3177. doi: 10.3390/ijms20133177
57. Yang Y, Tantai J, Sun Y, Zhong C, Li Z. Effect of hyperoside on the apoptosis of A549 human non-small cell lung cancer cells and the underlying mechanism. Mol Med Rep. (2017) 16:6483–8. doi: 10.3892/mmr.2017.7453
58. Mesas C, Martínez R, Ortíz R, Galisteo M, Prados J. Antitumor effect of the ethanolic extract from seeds of euphorbia lathyris in colorectal cancer. Nutrients. (2021) 13:566. doi: 10.3390/nu13020566
59. Eltamany EE, Elhady SS, Ahmed HA, Badr JM, Nafie MS. Chemical profiling, antioxidant, cytotoxic activities and molecular docking simulation of carrichtera annua DC. (Cruciferae). Antioxidants. (2020) 9:1286. doi: 10.3390/antiox9121286
60. Wang YP. Anti Aging Activity of Raspberry Glycoprotein GP3 and its Regulation on Klotho Gene Expression. Zhengzhou: Zhengzhou University (2018).
61. Chen Q, Ke L, Xiaoqing T, Liu S-C, Lin J-T, Hu C-C, et al. Phenolic components and antioxidant activity of fruits, stems and leaves of east China raspberry. Food Sci. (2020) 41:209–15.
62. Xia X, Li K, Ding Y, et al. Effects of Raspberry and it's effective position on learning and memory impairment rats of natural aging. Pharmacol Clin Chin Materia Medica. (2015).
63. Song B, Zheng B, Li T, Liu RH. Raspberry extract promoted longevity and stress tolerance via the insulin/IGF signaling pathway and DAF-16 in Caenorhabditis elegans. Food Funct. (2020) 11:3598–609. doi: 10.1039/C9FO02845E
64. Andreux PA, Blanco-Bose W, Ryu D, Burdet F, Rinsch C. The mitophagy activator urolithin A is safe and induces a molecular signature of improved mitochondrial and cellular health in humans. Nat Metab. (2019) 1:595–603. doi: 10.1038/s42255-019-0073-4
65. Corrêa WR, Serain AF, Netto LA, Marinho JVN, Salvador MJ. Anti-Inflammatory and antioxidant properties of the extract, tiliroside, and patuletin 3-O- β -D-Glucopyranoside from Pfaffia townsendii (Amaranthaceae). Evidencebased Complement Alternative Med. (2018) 2018:1–9. doi: 10.1155/2018/6057579
66. Li X, Tian Y, Wang T, Lin Q, Feng X, Jiang Q, et al. Role of the p-coumaroyl moiety in the antioxidant and cytoprotective effects of flavonoid glycosides: comparison of astragalin and tiliroside. Molecules. (2017) 22:1165. doi: 10.3390/molecules22071165
67. Velagapudi R, Aderogba M, Olajide OA. Tiliroside, a dietary glycosidic flavonoid, inhibits TRAF-6/NF-κB/p38-mediated neuroinflammation in activated BV2 microglia. BBA Gen Subjects. (2014) 1840:3311–19. doi: 10.1016/j.bbagen.2014.08.008
68. Chatzigeorgiou S, Thai QD, Tchoumtchoua J, Tallas K, Trougakos IP. Isolation of natural products with anti-ageing activity from the fruits of Platanus orientalis. Phytomedicine. (2017) 33:53–61. doi: 10.1016/j.phymed.2017.07.009
69. Derosa G, Maffioli P, Sahebkar A. Ellagic acid and its role in chronic diseases. Adv Exp Med Biol. (2016) 928:473–9. doi: 10.1007/978-3-319-41334-1_20
70. García-Ni?O W, Zazueta C. Ellagic acid: Pharmacological activities and molecular mechanisms involved in liver protection. Pharmacol Res. (2015) 97:84–103. doi: 10.1016/j.phrs.2015.04.008
71. Hseu Y-C, Chou C-W, Kumar KJS, Fu K-T, Wang H-M, Hsu L-S, et al. Ellagic acid protects human keratinocyte (HaCaT) cells against UVA-induced oxidative stress and apoptosis through the upregulation of the HO-1 and Nrf-2 antioxidant genes. Food Chem Toxicol. (2012) 50:1245–55. doi: 10.1016/j.fct.2012.02.020
72. Heijnen CGM, Haenen GRMM, Minou Oostveen R, Stalpers EM, Bast A. Protection of flavonoids against lipid peroxidation: the structure activity relationship revisited. Free Radical Res Commun. (2002) 36:575–81. doi: 10.1080/10715760290025951
73. Arts M, Dallinga JS, Voss HP, Haenen G, Bast A. A new approach to assess the total antioxidant capacity using the TEAC assay. Food Chem. (2004) 88:567–70. doi: 10.1016/j.foodchem.2004.02.008
74. Zhou Z-M, Yan D-M, Wang Y-K, Zhang T, Xiao X-R, Dai M-Y, et al. Discovery of quality markers in Rubus chingii Hu using UPLC-ESI-QTOF-MS. J Pharmaceut Biomed Anal. (2021) 203:114200. doi: 10.1016/j.jpba.2021.114200
75. Xiang T, Tong X, Li J, Xu Z, Li C. The effect of rhizosphere soil on the flavonoid metabolism in the roots of Tetrastigma hemsleyanum. (2019). doi: 10.21203/rs.2.17394/v1
76. Ali MS, Ibrahim SA, Jalil S, Choudhary MI. Ursolic acid: a potent Inhibitor of superoxides produced in the cellular system. Phytotherapy Res. (2007) 21:558–61. doi: 10.1002/ptr.2108
77. Salau VF, Erukainure OL, Ayeni G, Ibeji CU, Islam MS. Modulatory effect of ursolic acid on neurodegenerative activities in oxidative brain injury: An ex vivo study. J Food Biochem. (2021) 45:e13597. doi: 10.1111/jfbc.13597
78. Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism. (2018) 92:82–97. doi: 10.1016/j.metabol.2018.11.014
79. Fan BL, Gong CR, Sun FZ. Effects of hubei Rubus chingii Hu leaf on serum lipid metabolism in hyperlipidemia rats and human adults. Food Science. (2007) 28:526–9.
80. Zhang X-Y, Li W, Wang J, Li N, Cheng M-S, Koike K. Protein tyrosine phosphatase 1B inhibitory activities of ursane-type triterpenes from Chinese raspberry, fruits of Rubus chingii. Chin J Natural Med. (2019) 17:15–21. doi: 10.1016/S1875-5364(19)30004-4
81. Ke H, Bao T, Chen W. Polysaccharide from Rubus chingii Hu affords protection against palmitic acid-induced lipotoxicity in human hepatocytes. Int J Biol Macromolecules. (2019) 133:1063–71. doi: 10.1016/j.ijbiomac.2019.04.176
82. Fatima N, Hafizur RM, Hameed A, Ahmed S, Nisar M, Kabir N. Ellagic acid in Emblica officinalis exerts anti-diabetic activity through the action on β-cells of pancreas. Eur J Nutr. (2015) 56:591–601. doi: 10.1007/s00394-015-1103-y
83. Wang L, Wei Y, Ning C, Zhang M, Yang Y. Ellagic acid promotes browning of white adipose tissues in high-fat diet-induced obesity in rats through suppressing white adipocyte maintaining genes. Endocrine J. (2019) 66:467. doi: 10.1507/endocrj.EJ18-0467
84. Li X, Wei T, Wu M, Chen F, Zhang P, Deng Z-Y, et al. Potential metabolic activities of raspberry ketone. J Food Biochem. (2022) 46:e14018. doi: 10.1111/jfbc.14018
85. Fotschki B, Jukiewicz J, Jurgoński A, Sójka M. Fructo-oligosaccharides and pectins enhance beneficial effects of raspberry polyphenols in rats with nonalcoholic fatty liver. Nutrients. (2021) 13:833. doi: 10.3390/nu13030833
86. Su H, Xie L, Xu Y, Ke H, Bao T, Li Y, et al. Pelargonidin-3-O-glucoside Derived from Wild raspberry exerts antihyperglycemic effect by inducing autophagy and modulating gut microbiota. J Agric Food Chem. (2020) 68:13025–37. doi: 10.1021/acs.jafc.9b03338
87. Kalaycioglu Z, Uzaşçi S, Dirmenci T, Erim FB. α-Glucosidase enzyme inhibitory effects and ursolic and oleanolic acid contents of fourteen Anatolian Salvia species. J Pharmaceutical Biomed Anal. (2018) 155:284–87. doi: 10.1016/j.jpba.2018.04.014
88. Sithenkosi M. Ursolic acid and its derivatives as bioactive agents. Molecules (Basel, Switzerland.). (2019) 24:2751. doi: 10.3390/molecules24152751
89. Xing YS, Yi-Yan WU, Liang QC, et al. Effect of raspberry extract on norepinephrine of hippocampus in rats with ethanol withdrawal. Acta Chin Med Pharmacol. (2018).
90. Han B, Lin H, Chen J, Yi-Qun YU, Cao YB, Jiang YY. Preliminary screening active constituent against Candida albicans resistant to fluconazole from Rubus chingii Hu fruits. Chin J Mycol. (2012).
91. Bing XU, Cong-Ke Z. Study on the effect of removing chloasma of Raspberry extract. China Pract Med. (2012).
92. Stefan N, H?Ring HU, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. (2013) 1:152–62. doi: 10.1016/S2213-8587(13)70062-7
93. Xu W, Zhao M, Fu X, Hou J, Hu S. Molecular mechanisms underlying macrophage immunomodulatory activity of Rubus chingii Hu polysaccharides. Int J Biol Macromol. (2021) 185:24. doi: 10.1016/j.ijbiomac.2021.07.024
94. Ji YB, Bao XW, Shan Y, Yang SX, Cao L. Protective effect of raspberry extract on ConA-induced acute liver injuryin mice. China J Chin Materia Medica. (2019).
95. Tang XQ, Liu Y, Sun FZ, Fu SH, Yang WX. Toxicological Evaluation of Hubei R. Chingii Hu. Teratogenesis Carcinogenesis Mutagenesis. (2007) 19:395–98.
96. Zeng H, Ran Y, Lei L, Wang Y. Total flavonoid content, the antioxidant capacity, fingerprinting and quantitative analysis of fupenzi (Rubus chingii Hu.). Chin Med. (2015) 6:204–13. doi: 10.4236/cm.2015.64023
97. Sun D, Duan F, Jiang J, Liao X, Xiang Z, Sumei LI. Simultaneous determination of five flavonoid contents from the different drying process of Saururus chinensis (Lour.) Baill. by UPLC. J Guangdong Pharmaceut Univer. (2019).
98. Ma YJ, Bai WT, Zhu XF. Simultaneous determination of four flavonoids in Rubus chingii by MIPs-HPLC. Chin Tradit Patent Med. (2017) 039:2097–101.
99. Xu J, Chao J, Dai Y-T, Zhu C, Li Q, Takehisa T, et al. Quality evaluation of standard decoction of forsythiae fructus. Chin J Exp Tradit Med Formulae. (2018) 43:868–72. doi: 10.19540/j.cnki.cjcmm.20171023.008
100. Ping YH, Cai-Li LI, Xie YH. Determination of tiliroside and kaempferol in Rubus Chingii Hu. Food Res Dev. (2016).
101. CP Commission. Pharmacopoeia of the People's Republic of China, 2015. (2015). doi: 10.2753/CLG0009-4609430304
103. Ren YH, Jiang YY, Ge-Ge WU. Processing technology of raspberry Honey Jelly. J Anhui Agric Sci. (2016) 44. doi: 10.13989/j.cnki.0517-6611.2016.16.026
104. Xiang Y, Zang H, Guo L, Xiang Y, Yin L. Processing technology of canned raspberry. J Beihua Univer. (2018).
106. Sun J, Zhu H, Biology DO. Study on the production of raspberry beer. Liquor-Mak Sci Technol. (2014).
107. Huang BB. Research on production technology for wolfberry and raspberry fruit vinegar. Food Ind. (2016).
108. Ruimin L, Shuisheng Y, Liling Y, Zhangping Z, Qiuhua F, Rubin C. Current status of raspberry breeding and product development. Agric Serv. (2019) 36:67–69+71. doi: 10.19540/j.carolcarrollnkiCJCMM.20201224.101
Keywords: Rubus chingii Hu, bioactive components, pharmacological effects, quality control, drug development
Citation: He B, Dai L, Jin L, Liu Y, Li X, Luo M, Wang Z and Kai G (2023) Bioactive components, pharmacological effects, and drug development of traditional herbal medicine Rubus chingii Hu (Fu-Pen-Zi). Front. Nutr. 9:1052504. doi: 10.3389/fnut.2022.1052504
Received: 24 September 2022; Accepted: 12 December 2022;
Published: 09 January 2023.
Edited by:
Baiyi Lu, Zhejiang University, ChinaReviewed by:
Guangyang Liu, Insititute of Vegetables and Flowers (CAAS), ChinaZhiqiang Wang, Hebei University, China
Copyright © 2023 He, Dai, Jin, Liu, Li, Luo, Wang and Kai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guoyin Kai,  guoyinkai1@126.com
guoyinkai1@126.com
†These authors have contributed equally to this work and share first authorship
 Beihui He
Beihui He Linghao Dai1†
Linghao Dai1†  Li Jin
Li Jin Minmin Luo
Minmin Luo Guoyin Kai
Guoyin Kai