- 1Laboratory of Physiopathology, Molecular Genetics and Biotechnology, Faculty of Sciences Ain Chock, Health and Biotechnology Research Centre, Hassan II University of Casablanca, Casablanca, Morocco
- 2Department of Drug Analysis, “Iuliu Hațieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania
- 3Department of Food Science, University of Agricultural Science and Veterinary Medicine of Cluj-Napoca, Cluj-Napoca, Romania
- 4Molecular Nutrition and Proteomics Lab, CDS3, Life Science Institute, University of Agricultural Science and Veterinary Medicine of Cluj-Napoca, Cluj-Napoca, Romania
- 5Clinical Center of Diabetes, Nutrition and Metabolic Diseases, “Iuliu Haţieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania
- 6Department of Molecular Sciences, “Iuliu Hațieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania
- 7Cognitive Neuroscience Laboratory, Department of Psychology, Babeș-Bolyai University, Cluj-Napoca, Romania
- 8Internal Medicine Department, 4th Medical Clinic “Iuliu Haţieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania
- 9Food Biotechnology and Molecular Gastronomy, CDS7, Life Science Institute, University of Agricultural Science and Veterinary Medicine of Cluj-Napoca, Cluj-Napoca, Romania
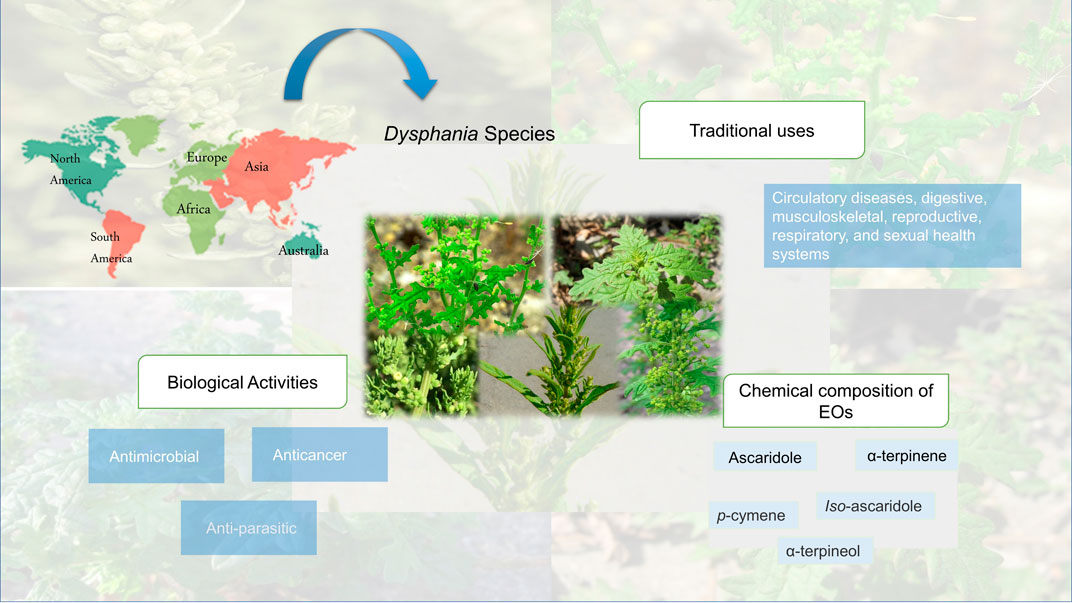
The genus Dysphania belongs to the Amaranthaceae family and is known for its many health benefits. Therefore, it is commonly available worldwide and includes more than 47 species, five species have been mainly reported, and D. ambrosioides has been one of the most widely used plants for thousands of years as a remedy for a wide range of ailments. In recent investigations, the essential oils of the genus Dysphania have been examined for their antibacterial, antioxidant, and antiviral properties related to specific components such as terpenoid compounds that exhibit pharmacological activity. Moreover, some of Dysphania’s compounds show a toxicological effect. Therefore, the objective of the study was to provide EO chemical composition and pharmacological data of the genus Dysphania.
Introduction
Since antiquity, natural molecules from various sources have been used to cure human ailments (Hassan et al., 2012; Murray et al., 2013; Kola-Mustapha et al., 2020). Among the most significant biomolecule sources are the derivatives of aromatic medicinal plants. As a result, multiple studies have shown that bioactive chemicals from plants have a promising benefic health effect. Among these is the Amaranthaceae family, which is distinguished by the diversity of produced secondary metabolites. This family contains over 175 genera and 2,000 herb species (Mroczek, 2015). The genus Dysphania is known for its many pharmacological and preclinical properties. Hence, it is commonly available worldwide and includes more than 47 species (Kim et al., 2019).
D. ambrosioides is known as one of the most important species of the Dysphania genus, used in the food, cosmetic, and pharmaceutical industries, and also used in traditional medicine to treat several foods (Hallala et al., 2010; Kasali et al., 2021), followed by Dysphania botrys (syn. Chenopodium botrys), which represents the second species most studied in the literature (Morteza-Semnani, 2015) Dysphania multifida, Dysphania schraderiana, and Dysphania pumilio are still less studied. The chemical composition of Dysphania essential oils (EOs) depends on different environmental factors (Barra, 2009). However, the composition of all the EO examined was different, with a significant quantity of monoterpene compounds (Brahim et al., 2015; Zefzoufi et al., 2020). Dysphania EO is also antibacterial (Kandsi et al., 2022), antifungal (Chekem et al., 2010), anti-oxidant (Villalobos-Delgado et al., 2020), and antiviral (Arena et al., 2018).
To the best of our knowledge, there are no reports in the literature that provide a comprehensive analysis of Dysphania species. In an effort to better understand its current research status and justify the further exploration and comprehensive application of this genus, we review the botanical, ethnopharmacological, chemical composition, and pharmacological activities of Dysphania spp., in addition to its distribution and its possible mechanisms of action and toxicology.
Methodology
We searched for published articles and grey literature (e.g., unpublished studies, theses, reports, and conference abstracts) that fit these two search criteria: 1. Original research articles with hypothesis tested in the laboratory (e.g., in vitro, in vivo, preclinical studies) assessing the essential oils’ biological activities and toxicology of the Dysphania genus, and 2. studies published in English with full *pdf files available. There were no restrictions on the publication dates of the selected papers, which included both contemporary and older works, to collect extensive data for the review. Using Science Direct, PubMed, ResearchGate, Google Scholar, and Web of Science (WOS: 22 July 2022 with University Hassan II of Casablanca institutional subscription), we found 1,000 publications (Figure 1) with this keyword search: ((Dysphania) AND (ethnopharmacology OR pharmacology*) (activity* OR bio* activity) AND (toxicology*)).
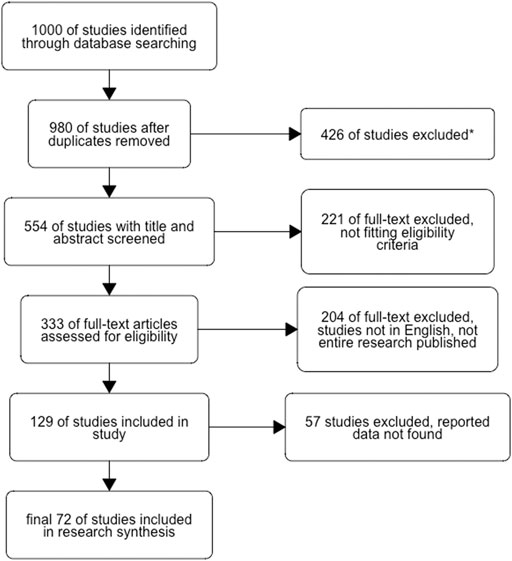
FIGURE 1. Flowchart of the study design and the bibliographic sources selection process. The search protocol using keywords selection (EO of Dysphania chemical composition, bioactivity, and toxicity) resulted in 1,000 publications; 20 duplicates were removed; 426 studies were excluded due to the presence of abstract, citations, and thesis; 221 full-text excluded not fitting eligibility criteria with the topic field out of our study aim, 204 were excluded when not the entire research published, studies not in English, and 57 studies were excluded because the data reported have been not founded. The figure was done using R metagear package.
This paper has chosen, evaluated, and discussed a few selected publications. After duplicate removal, excluded studies that were not in our specific aim, and excluded reports resulted in 333 studies.
We established the requirements for the studies’ selection; articles with extensive studies on the Dysphania essential oils (EOs) composition, therapeutic uses, biological, and pharmacological activities, as well as toxicity, were eligible for inclusion. The exclusion criteria were as follows: if the topic field is not our aim, not the entire research has been published, and studies that were not published in English. We found the essential data/results/papers on the subject, which resulted in 129 publications included in the screening, from which 57 have only an abstract or the title, with no available *pdf files. We conducted the selection procedure for the most relevant articles for this research based on this selected article.
Dysphania genus
Currently, Dysphania genus belongs to the new classification, which aggregates the Chenopodiaceae-Amaranthaceae in a single-family known as Amaranthaceae according to the APG III system (Group, 2009), this genus comprises more than 47 species. The representatives of the genus are mainly ruderal and weed plants, more common in the tropics, subtropics, and warm-temperate zones (Judd and Ferguson, 1999; Sukhorukov et al., 2016). Five species have been reported in the literature; D. ambrosioides, D. botrys, D. multifida, D. schraderiana, and D. pumilio (Mosyakin and Clemants, 2002; The Plant List, 2020). The Dysphania species are known to generate glandular white hairs and yellow or orange subsessile glands. These glands contain essential oils that give off a distinctive aromatic odor that frequently remains in herbarium specimens for years (Uotila et al., 2021).
Distribution
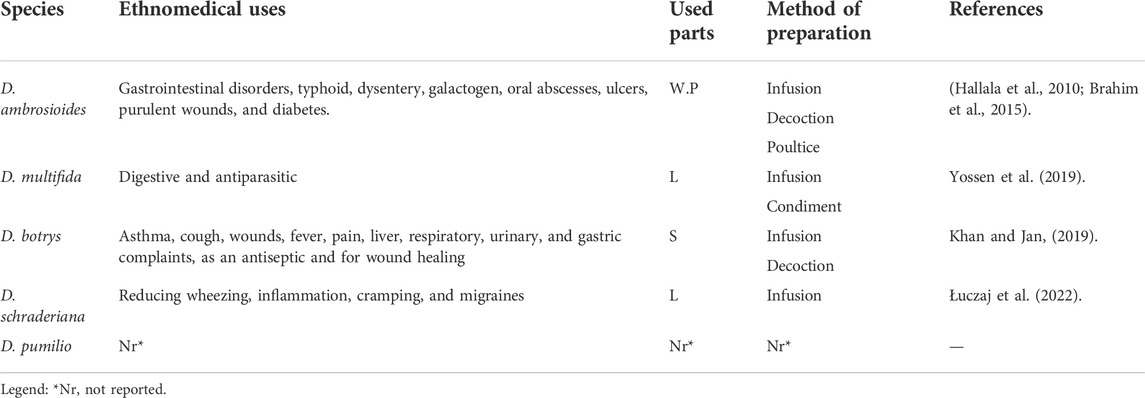
Dysphania Spp., are pervasively distributed throughout both temperate and tropical parts of the world. This genus became more widespread due to its ability to adapt to a variety of ecological conditions. There are two majors domesticated Dysphania, D. ambrosioides, and D. botrys. These two species have been cultivated over vast areas of the old world (Sukhorukov et al., 2016). Table 1 provides a list of the common Dysphania species distribution.
Botanical description
D. ambrosioides (L.) Mosyakin and Clemants, is the most well-known species from this genus, represents an annual or short-lived perennial herbaceous plant, with a strong odor, which reaches up to 1 m high, with erect stems, very branched, alternate leaves elongated with acute apex, edges serrated, hairy, of different sizes sessile; racemose inflorescence presenting small white flowers with 3–5 free or united sepals and 3 to 5 free or rarely adnate stamens, compressed spherical ovary and many black seeds (with a length less than 0.08 mm) (Sá et al., 2016; Paniagua-Zambrana et al., 2020).
D. botrys (L.) Mosyakin and Clemants, is a naturally growing wild plant, traditionally used by rural and endemic inhabitants, has a characteristic odor due to the presence of sesquiterpenes and monoterpenes, and is an annual plant of 20–50 cm, stem erect, angular, branching often from the base, with erect-spreading branches, lower leaves long petiolate, pinnately lobed, racemose inflorescence of a yellowish green (Khan and January 2019).
D. multifida (L.) Mosyakin and Clemants, commonly known as “paico”, is an aromatic plant widely used for medicinal purposes, perennial plant of 30–80 cm, pubescent, with a penetrating and pleasant smell, stems numerous, very branchy, leaves small, puberulous-glandulosa, shortly petiolate, with lanceolate or linear lobes, greenish (Yossen et al., 2019).
D. schraderiana (Schult.) Mosyakin and Clemants, this plant is used in a variety of applications such as medicine. Pubescent annual (height: 20–60 cm), oblong leaves (long: 2–6 cm, wide: 1.5–3.5 cm), attenuated base, obtuse to acuminate apex, pinnately lobed margins, glabrescent petiole (2–10 mm long). Flowers with 5 oval sepals (long: 1 mm), and 5 stamens, are grouped in inflorescence (Łuczaj et al., 2022).
D. pumilio (R.Br.) Mosyakin and Clemants, is one of the popular invasive species, pubescent annual (height: 5–45 cm). Leaves ovate to elliptical (length: 0.5–2.5 cm, width: 0.5–1.5 cm), wedge-shaped base, obtuse apex, entire margins, glabrescent petiole (3–15 mm) (see Figure 2). Flowers with 5 elliptical to oblong sepals (long: 1 mm), and 1 stamen sometimes absent, grouped in inflorescence (diameter: 2–3 mm) assembled in axillary cymes (length: 3–7 cm) (Bogosavljević and Zlatković, 2017).

FIGURE 2. Dysphania species. (A) D. ambrosioides, (B) D. botrys, (C) D. mutifida, (D) D. schraderiana, (E) D. pumilio.
Ethnopharmacology
Since ancient times, Dysphania species have been used around the world to cure various ailments (Table 2), specifically circulatory diseases, digestive, musculoskeletal, reproductive, respiratory, and sexual health systems (Bussmann et al., 2018). Aside from being utilized as an herbal remedy, some plants of this genus may be consumed due to their nutritional components. The leaves, fruits, and flowers can also be made into different food products. For example, they are used as spices in different countries (Barragán and Carpio, 2009; Barros et al., 2013). Traditional uses of Dyphania Spp., are represented in Table 2.
Chemical composition
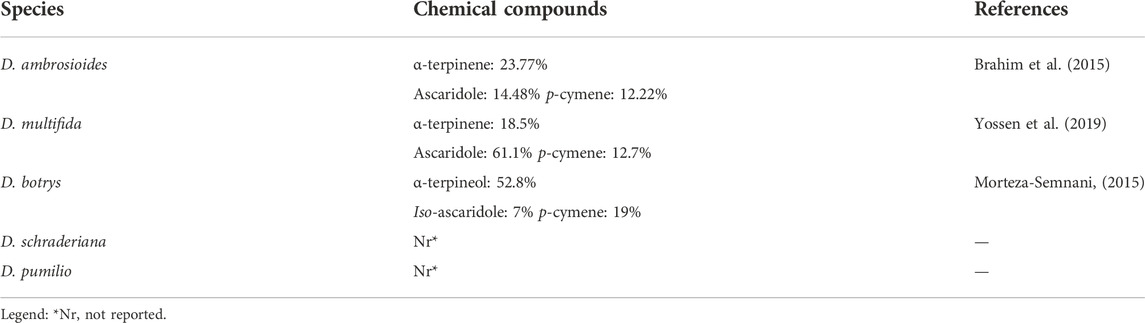
Several studies have revealed that Dysphania is an important genus with various compounds, especially essential oils. The most prevalent were monoterpenes, and sesquiterpenes (Kokanova-Nedialkova et al., 2009; Barros et al., 2013). Currently, approximately 45 terpenoid compounds have been reported and isolated from the fruits, seeds, leaves, and flowers of Dysphania species EO. The main chemical compounds occurring in the essential oils obtained from the Dysphania genus are represented in Table 3.
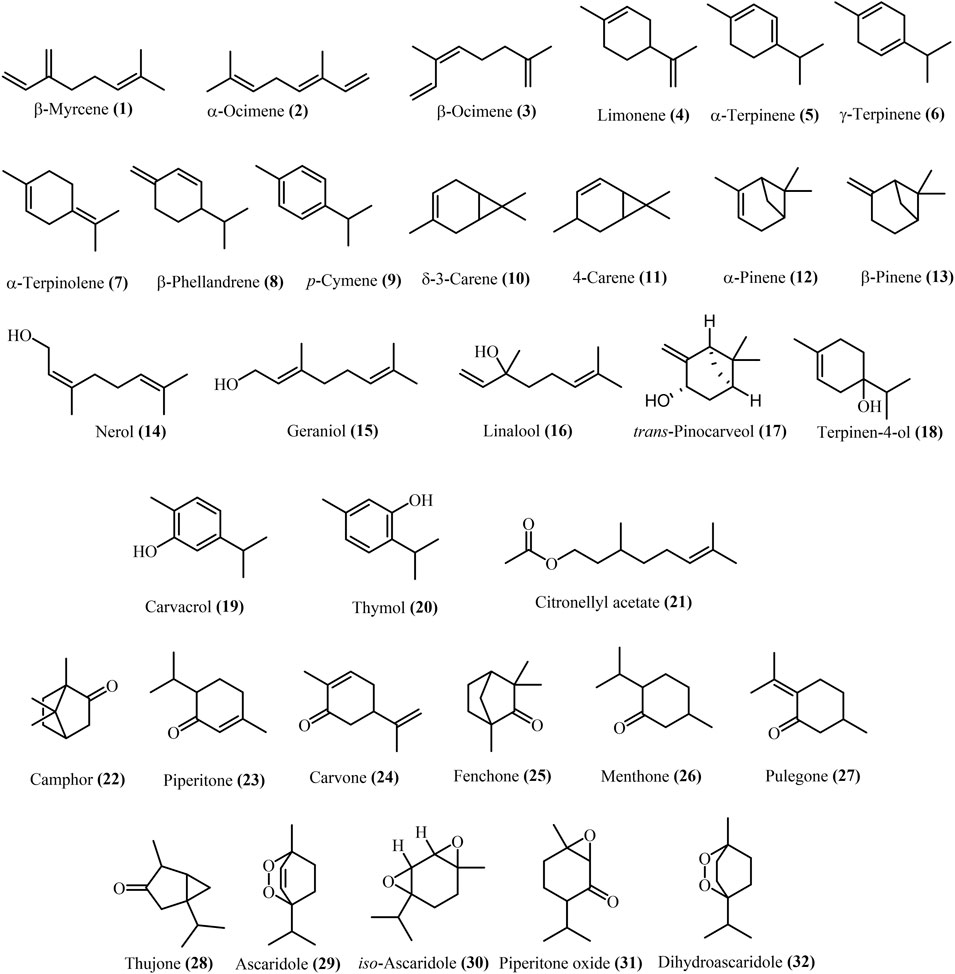
Approximately 44 papers covered the Dysphania EO assessment. The majority of the paper identified the components of D. ambrosioides EO are oxygenated monoterpenes. In several studies (Gupta et al., 2002; Boutkhil et al., 2009; Brahim et al., 2015; Bisht and Kumar, 2019), α-terpinene (5) was quantified as the main constituent in D. ambrosioides EO, while ascaridole (29) was reported as the most abundant components in D. multifida EO (Yossen et al., 2019). Less frequently, δ-3-carene (10), limonene (4), thymol (20), carvacrol (19), γ-terpinene (6), α-terpinolene (7), piperitone oxide (31), geraniol (15), α-pinene (12), β-pinene (26), iso-ascaridole (20), β-myrcene (1), α-ocimene (2), β-ocimene (3), citronellyl acetate (21), β-phellandrene (8), dihydroascaridole (32), trans-pinocarveol (17), carvone (24), piperitone (23) were reported in D. multifida and D. ambrosioides EO (Arena et al., 2018), while p-cymene (9), and 4-carene (11) were reported as main components of D. ambrosioides EO in another study (Zefzoufi et al., 2020). Other compounds, camphor (22), δ-3-carene (23), fenchone (25), linalool (16), menthone (26), nerol (14), β-pinene (13), pulegone (27), terpineol-4-ol (18), thujone (28), and iso-ascaridole (30) are represented in D. botrys EO. The structures of monoterpenes from 1 to 32 are shown in Figure 3.
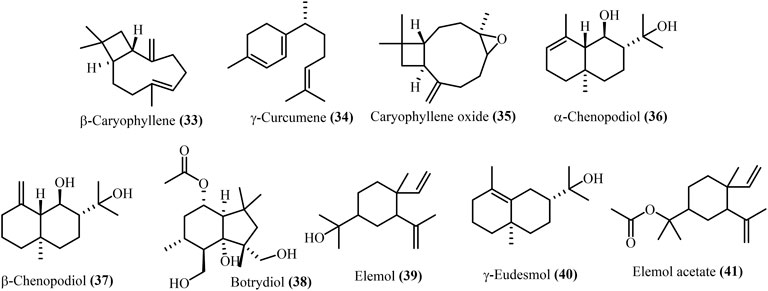
Major sesquiterpenes in D. ambrosioides included β-caryophyllene (33), γ-curcumene (34), and caryophyllene oxide (35) (Kokanova-Nedialkova et al., 2009). While, D. botrys included elemol (39), elemol acetate (41), α-chenopodiol (36), β-chenopodiol (37), botrydiol (38), and eudesmol (40) are shown in Figure 4. These main sesquiterpenes were identical across different Dysphania populations based on GC-MS data, although relative quantity varied (Pino et al., 2003; Singh et al., 2008).
In addition, many intrinsic and extrinsic factors, such as environmental factors, affect the D. ambrosioides essential oils yield and constituents. Plants may be stressed due to high or low salinity, causing a change in the content of EO (Verma and Shukla, 2015). According to several authors (de Carvalho et al., 2018b), the amount of four main volatile constituents (α-terpinene, p-cymene, E-ascaridole, and Z-ascaridole) is affected by salt concentrations. Salts are essential to plant growth and metabolism. High concentrations may be toxic (Mosa et al., 2017). The blue LED was also shown to block the production of ascaridole (29) (53.21%), whereas fluorescent light increased the conversion of α-terpinene (5) to ascaridole (29) (de Carvalho et al., 2020). In general, these results agree with the observation that many enzymes of the secondary pathways are light-dependent (Yabuta et al., 2007; Alvarenga et al., 2015). Another study (Yousefi et al., 2011) showed that the development stages of D. botrys are affected by heavy metals. Treatments without CaCl2 and MgSO4 had an antagonistic connection with p-cymene (9), and treatments with MgSO4 at 1,480 mg L−1 gave higher levels of ascaridole (19). KH2PO4 at a concentration of 680 mg L−1 caused an excess of ascaridole (29) to be found in the treatment. α-terpinene (5) represents a significant amount in treatment by CaCl2 at a concentration of 880 mg L−1 (de Carvalho et al., 2018a) ascaridole (29) content in the leaves increased when quail manure was used, whereas it increased in the inflorescences when chicken manure was used (Bibiano et al., 2019). However, the greatest α-terpinene (5) content was reported without using chitosan. According to the biosynthetic pathway, chitosan and salicylic acid favoured the conversion of α-terpinene (5) to ascaridole (29) (Dembitsky et al., 2008; de Carvalho et al., 2020). This paper mainly focused on C10 monoterpenes, and C15 sesquiterpenes for their importance. All these terpenoids are derived from two distinct biochemical pathways; the (MEP) 2C-methyl-D-erythritol-4-phosphate pathway, which is active in the plastids, begins from pyruvate and glyceraldehyde-3-phosphate, whereas the (MVA) mevalonic acid pathway active in the cytosol and starts from acetyl CoA (Bergman et al., 2019).
Health benefits
Antimicrobial effects
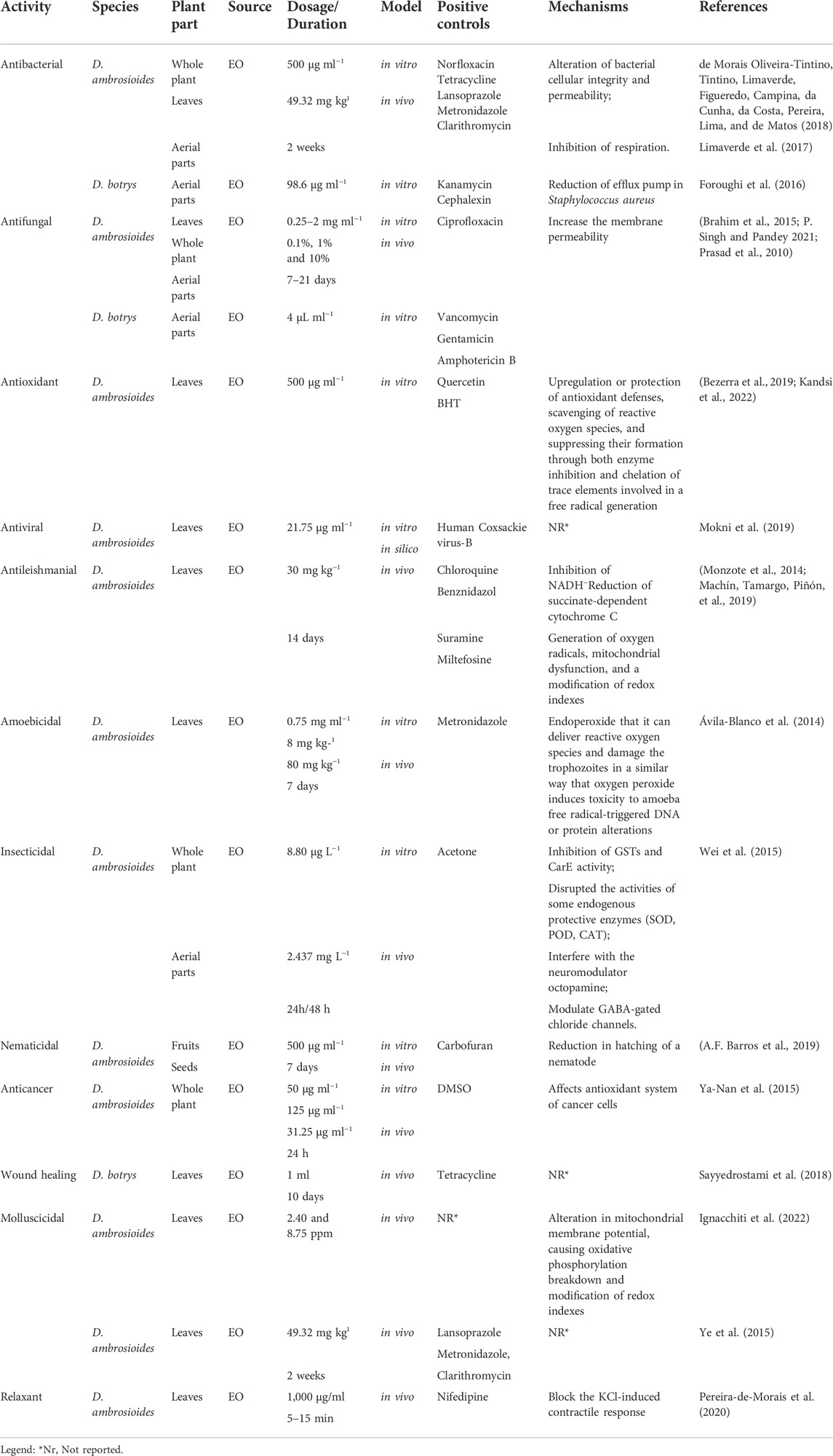
Bacteria have evolved several mechanisms to withstand antibiotic action. Several investigations have indicated that D. ambrosioides L. has inhibitory action against a wide spectrum of pathogenic bacteria. Brahim et al., 2015 (Brahim et al., 2015) reported that EO isolated from D. ambrosioides are more active against Bacillus cereus and Micrococcus luteus than Klebsiella pneumoniae and Pseudomonas aeruginosa with zones of inhibition ranging from 15.33 to 21.5 mm and from 7.17 to 19.17 mm, for Gram-positive and Gram-negative bacteria, respectively. The cell envelope structure explains this, since Gram-negative bacteria have an additional membrane, limiting hydrophobic compound diffusion. D. ambrosioides EO has also been shown to have antibacterial activity against Helicobacter pylori (Ye et al., 2015), also, against Escherichia coli, staphylococcus aureus, and Enterococcus faecalis (Kandsi et al., 2022) with ZI ranging from 9 to 24 mm. D. botrys EO also showed strong antimicrobial activity against a variety of bacteria (Staphylococcus aureus, Bacillus cereus, Staphylococcus saprophyticus, Klebsiella pneumoniae, Staphylococcus epidermidis, Streptococcus mutans, Listeria monocytogenes, and Salmonella typhimurium) with ZI ranging from (9–22 mm) (Foroughi et al., 2016). Numerous studies evaluated the antifungal activity of D. ambrosioides EO against fungal. Brahim et al., 2015 (Brahim et al., 2015) also reported high anticandidal activity, where Candida albicans was the most susceptible yeast, having the lowest minimum inhibitory concentration. Likewise, Mokni et al., 2019 (Mokni et al., 2019) observed that D. ambrosioides EO exhibited considerable antifungal activity against Candida albicans. Similarly, good activity was recorded for D. botrys EO on C. albicans and showed an inhibitory effect on Aspergillus species and Bacillus subtilis (Mahboubi et al., 2011), while for Trichophyton mentagrophytes, Epidermophyton floccosum, Candida albicans, Aspergillus niger, and Microsporum canis. D. botrys EO showed ZI ranging from (14–20 mm) (Tzakou et al., 2007). Available scientific data have shown consistent findings from several authors. The following main points have evolved as a result of this: These plants EO have good antimicrobial activity against a wide range of pathogens, including Gram-negative and Gram-positive bacteria and fungi, this high activity has been linked to the presence of monoterpene hydrocarbons (limonene (4), p-cymene (9), and ascaridole (29), thymol (20), carvacrol (19), and α-terpinene (5)). All mechanisms described in the literature show that Dysphania EO affects the cellular integrity of bacteria, a decrease in respiration, and an alteration in permeability. Few studies have described the antimicrobial activities from other Dysphania species (Table 4). The inhibitory effectiveness of Dysphania EO against microbial growth is stronger than reference antimicrobials even in experiments with the positive control, hence, EO from this species can be advised as a replacement for conventional antimicrobial agents. It should be noted that most research on the antibacterial properties of Dysphania spp. has been conducted in vitro, which does not ensure that the results would be the same in vivo. Furthermore, the susceptibility testing in the aforementioned research solely employed traditional techniques. However, additional techniques may be modified to determine the antimicrobial susceptibility of EO, including bioautography, flow cytometry, and bioluminescence experiments.
Antiviral effects
One of the common viruses is enteroviruses, specifically the Coxsackie B4 virus (CVB4) enteroviruses that belong to the Picornaviridae family, which is associated with serious illnesses, including myocarditis and meningoencephalitis (Sin et al., 2015). In this context, the EO obtained from D. ambrosioides L., growing wildly in Tunisia, demonstrated a significant antiviral effect against the CV-B4 virus. This activity could be attributed to ascaridole (29) (Mokni et al., 2019). However, more research in vitro and in vivo is needed to evaluate the antiviral activity of EO and their active compounds isolated from all Dysphania spp.
Anti-leishmanial effects
The hunt for effective therapeutics to treat Leishmaniasis has become an urgent requirement due to the absence of effective medicines and the limits of present treatments (Machín et al., 2019). The anti-leishmanial activity of D. ambrosioides was demonstrated by Monzote et al., 2014 and 2018 (Monzote et al., 2014; Monzote et al., 2018) against amastigotes and promastigotes of Leishmania amazonensis. Results show a more significant inhibitory effect of ascaridole (29). This effect is by reducing succinate-dependent cytochrome C due to the inhibition of NADH. To more understand the effects of D. ambrosioides EO and resolve the stability and solubility problems of EO, some studies (Machín et al., 2019) aim to explore the encapsulation of D. ambrosioides L. EO in nanocochleates (lipid-based delivery system) and investigated in vitro and in vivo against L. amazonensis. The results showed that D. ambrosioides L. EO-nanocochleates (NC) did not affect the EOs’ in vitro inhibitory efficacy. The formulation caused no mortality or weight loss higher than 10% in the animal model (Table 4). Mice treated with D. ambrosioides EO-NC had more extensive lesions than those treated with EO. This activity may be related to the presence of some terpenoid compounds. Hence, the results showing a potent anti-leishmanial activity from in vitro and in vivo indicating a safe application as drug.
Antioxidant effects
Several studies showed that D. ambrosioides L. EO had an essential antioxidant activity. Santiago et al., 2016 (Santiago et al., 2016) reported this activity by different methods DPPH and β-carotene/linoleic acid, showed higher activity of EO in the β-carotene/linoleic acid test. Also, Brahim et al., 2015 (Brahim et al., 2015) demonstrated that D. ambrosioides L. EO exhibits free radical scavenging activity by using the DPPH test. Indeed, they found the highest antioxidant capacity by inhibiting lipid peroxidation via a β-Carotene/linoleic acid bleaching test. Also, Brahim et al., 2015 (Brahim et al., 2015) marked the high activity by reducing potency (Table 4). The potent antioxidant activity of Dysphania EO can be due to its high content of α-terpinene (5), which is characterized by its powerful antioxidant capacity that is probably attributed to the presence of strongly activated methylene groups (Table 4). However, the in vitro assays for measuring antioxidant activity have little pharmacological significance and only partially validate the biological impact, more studies in vivo about oxidative stress are needed.
Anticancer effects
Some studies demonstrate the cytotoxic activity of D. ambrosioides L. EO against tumours; the authors have demonstrated that the EO reduced cell growth and were cytotoxic to human breast cancer cell lines MCF-7 in a dose and time-dependent manner, via an apoptosis-related mechanism. D. botrys EO showed maximum growth inhibitions against the A549 cell line and inhibited the growth of the MCF-7 cell line (Table 4) (Shameem et al., 2019). The reported research has shown the precise anti-tumor mechanisms of D. ambrosioides EO, which are related to apoptosis induction (Table 4). Therefore, these results could offer an actual overview on the effects of Dysphania EO on tumoural cells. However, in these investigations, the cytotoxic effects of Dysphania EO were only assessed in tumour cell lines. There have not been any human clinical trials to look at the pharmacokinetics and therapeutic effects of EO and their compounds on cancer patients. Clinical investigations involving humans and animal models should be the main topics of future study. Moreover, further studies to elucidate the antitumoral effect are required.
The benefic effect of Dysphania EO (antimicrobial, antiviral, antifungal, antileishmanial, insecticidal, nematocidal, antioxidant, antitumoral, anti-ulcer, and relaxant) are sown in Table 4.
Toxicology
The centuries-old use of medicinal plants has shown that some of these plants contain potentially dangerous substances (Ndhlala et al., 2013). D. ambrosioides L. is one of the plants described as having a toxicological risk, specially indicated for essential oils (GUYTON, 1946).
Several species, including D. botrys, and D. ambrosioides possess compounds that have been demonstrated to interfere with mitochondrial function (Nagle et al., 2011). The toxicity of EO obtained from Dysphania can be associated with the presence of some major components, carvacrol (19), caryophyllene oxide (35), and ascaridole (29), which induce suppression of the respiratory function in the mitochondria, or in the complex I of the mitochondrial electron transport chains (Figure 5) (Monzote et al., 2018), this toxic effect emerging on the kidneys, liver, and intestine (Derraji et al., 2014). Nevertheless, in a recent study by Li et al., 2020 (Li et al., 2020), dose-dependent toxicity was demonstrated in mice, providing some support for using the EO in a safe way in traditional medicine. However, their utilization is contraindicated during pregnancy and breastfeeding for infants under three, and adult patients who are distressed or suffer from liver or renal illnesses (Potawale et al., 2008).
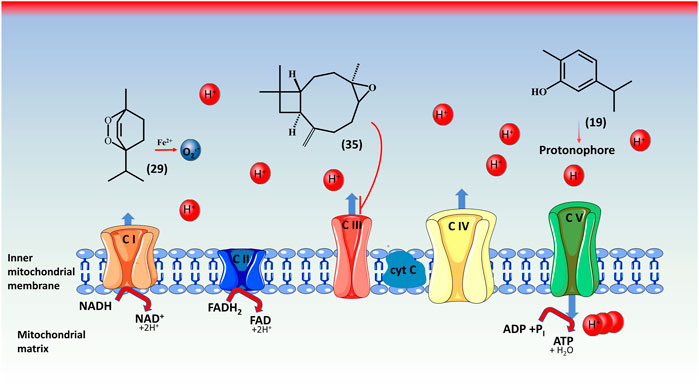
FIGURE 5. Toxicity mechanisms of ascaridole (29), carvacrol (19), and caryophyllene oxide (35) in mitochondria. Both oxidative stress and mitochondrial dysfunction are employed in the mechanism of toxicity by the Dysphania’s EO. The EO have inhibitory effects on mitochondria’s ETC (electron transport chain) complex I-III. Caryophyllene oxide (35) carries out inhibition on complex III (CIII). Ascardiole (29) following activation by iron (Fe2+) threatens mitochondrial uncoupling and triggers superoxide radical formation (O2.-). Carvacrol (19) has no direct inhibiting effects, but a synergistic effect with ascaridole. *complex I: NADH ubiquinone oxidoreductase; complex II: succinate ubiquinone oxidoreductase; complex III: ubiquinol cytochrome c oxidoreductase; complex IV: cytochrome C oxidase; complex V: F1F0ATP synthase. NADH, nicotinamide adenine dinucleotide hydrogen; NAD+, nicotinamide adenine dinucleotide; FADH2, flavin adenine dinucleotide (hydroquinone form); FAD, flavin adenine dinucleotide; H+, protons; H2O, water; H, hydrogen; O, oxygen; Fe, iron. The figure was produced using Servier Medical Art.
The toxicity of ascaridole (29) was observed by activation in the presence of iron, which allows it to be more toxic, resulting in carbon-centred radicals, which are very reactive and can initiate lipid peroxidation and reduce respiration. Caryophyllene oxide (35) is the principal generator of superoxide radicals and directly influences complex III. Carvacrol (19) reacts as a protonophore and does not have a direct physiological effect. All these actions induce a decrease in ATP production and an increase in superoxide radicals.
Conclusions and further perspectives
The present review offers the first insights of selected literature regarding the chemical composition of Dysphania EOs, their pharmacological properties, and their applications in traditional medicine. Plants of this genus have been used since ancient times to treat many diseases, and these properties have been confirmed by numerous pharmacological studies.
Distinctive chemical constituents have been isolated and identified as belonging to different species. Indeed, the literature has shown that the main components of these essential oils are α-terpinene, ascaridole, iso-ascaridole, α-terpineol, and p-cymene. Overall, these compounds can change due to abiotic and biotic factors that affect essential oil content and yield. Most chemical studies have focused on the EO content of D. ambrosioides, D. multifida, and D. botrys, while further research on the chemical composition of the EO of other species is needed in order to determine their chemical composition. Determining the bioactivity of other volatile compounds from all species of Dysphania would be critical for future investigation and its impact on health. Previous research has revealed the extensive medicinal applications of volatile compounds from different botanical parts of Dysphania spp. (seeds, fruits, and leaves) in a range of in vitro and in vivo test models. Dysphania spp. EOs have been demonstrated to possess antibacterial, antifungal, antioxidant, anti-cancer, antiviral, antileishmanial, amoebicidal, and anti-inflammatory properties, and lastly, nematocidal, and insecticidal activities at different doses/concentrations. The chemical composition and pharmacological results validate and support some ethnopharmacological uses of Dysphania spp. in traditional medicine.
As this review shown, the Dysphania genus EOs, rich in secondary metabolites and various biological activities, can constitute an alternative to certain synthetic drugs to bring health benefits to human diseases in the future. However, according to the literature, current knowledge of Dysphania species contains several gaps that require further investigation in preclinical and clinical studies.
Author contributions
Conceptualization, AD, SCH, and RS; Funding acquisition, DV and OP; Writing—Original Draft Preparation, AD and SCH; Visualization, RV and RS; Writing—Review and Editing, AF, AC, RV, and OA; Supervision, BEK, RS, and AS. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Romanian Ministry of Education and Research, CCCDI—UEFISCDI, project number PN-III-P4-ID-PCE-2020-2126, within PNCDI III.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
A.P, Aerial parts; CarE, Carboxylesterase; CAT, catalase; DPPH, 2,2-diphenyl-1-picrylhydrazyl; EO, essential oils; F, Fruits; GST, glutathione-S-transferase; IC50, 50% inhibitory concentration; L, Leaves; MIC, Minimal Inhibition Concentration; POD, Peroxidase; S, Seeds; SOD, superoxide dismutase; W.P, Whole plant; ZI, Zones of inhibition.
References
Alvarenga, I. C. A., Pacheco, F. V., Silva, S. T., Bertolucci, S. K. V., and Pinto, J. E. B. P. (2015). In vitro culture of Achillea millefolium L.: Quality and intensity of light on growth and production of volatiles. Plant Cell Tiss. Organ Cult. 122 (2), 299–308. doi:10.1007/s11240-015-0766-7
Arena, J. S., Omarini, A. B., Zunino, M. P., Peschiutta, M. L., Defagó, M. T., and Zygadlo, J. A. (2018). Essential oils from Dysphania ambrosioides and Tagetes minuta enhance the toxicity of a conventional insecticide against Alphitobius diaperinus. Industrial Crops Prod. 122, 190–194. doi:10.1016/j.indcrop.2018.05.077
Ávila-Blanco, M. E., Rodríguez, M. G., Moreno Duque, J. L., Muñoz-Ortega, M., and Ventura-Juárez, J. (2014). Amoebicidal activity of essential oil of Dysphania ambrosioides (L.) Mosyakin & Clemants in an amoebic liver abscess hamster model. Evidence-Based Complementary Altern. Med. 2014, 930208. doi:10.1155/2014/930208
Barra, A. (2009). Factors affecting chemical variability of essential oils: A review of recent developments. Nat. Product. Commun. 4 (8), 1934578X0900400–1154. doi:10.1177/1934578x0900400827
Barragán, Á., and Carpio, C. (2009). “Introducción,” in Plantas como alimento de invertebrados útiles, 76–79.
Barros, A. F., Campos, V. P., de Paula, L. L., Oliveira, D. F., de Silva, F. J., Terra, W. C., et al. (2019). Nematicidal screening of essential oils and potent toxicity of Dysphania ambrosioides essential oil against Meloidogyne incognita in vitro and in vivo. J. Phytopathol. 167 (7-8), 380–389. doi:10.1111/jph.12803
Barros, L., Pereira, E., Calhelha, R. C., Dueñas, M., Carvalho, A. M., Santos-Buelga, C., et al. (2013). Bioactivity and chemical characterization in hydrophilic and lipophilic compounds of Chenopodium ambrosioides L. J. Funct. Foods 5 (4), 1732–1740. doi:10.1016/j.jff.2013.07.019
Bezerra, J. W. A., Costa, A. R., de Freitas, M. A., Rodrigues, F. C., de Souza, M. A., da Silva, A. R. P., et al. (2019). Chemical composition, antimicrobial, modulator and antioxidant activity of essential oil of Dysphania ambrosioides (L.) Mosyakin & Clemants. Comp. Immunol. Microbiol. Infect. Dis. 65, 58–64. doi:10.1016/j.cimid.2019.04.010
Bibiano, C. S., de Carvalho, A. A., Bertolucci, S. K. V., Torres, S. S., Corrêa, R. M., and Pinto, J. E. B. P. (2019). Organic manure sources play fundamental roles in growth and quali-quantitative production of essential oil from Dysphania ambrosioides L. Industrial Crops Prod. 139, 111512. doi:10.1016/j.indcrop.2019.111512
Bisht, B. S., and Kumar, A. (2019). Terpenoid composition of Chenopodium ambrosioides L . and its antimicrobial activity from uttarakhand. Himalaya India 9, 612–617. doi:10.22270/jddt.v9i4-A.3536
Bogosavljević, S., and Zlatković, B. (2017). Dysphania pumilio (R. Br.) Mosyakin & Clemants (Amaranthaceae), a new allochthonous species in the flora of Serbia. Bot. Serbica 41 (1), 83–87. doi:10.5281/zenodo.455155
Boutkhil, S., El Idrissi, M., Amechrouq, A., Chbicheb, A., Chakir, S., and El Badaoui, K. (2009). Chemical composition and antimicrobial activity of crude, aqueous, ethanol extracts and essential oils of Dysphania ambrosioides (L.) Mosyakin & Clemants. Acta bot. gallica 156 (2), 201–209. doi:10.1080/12538078.2009.10516151
Brahim, M. A. S., Fadli, M., Hassani, L., Boulay, B., Markouk, M., Bekkouche, K., et al. (2015). Chenopodium ambrosioides var. ambrosioides used in Moroccan traditional medicine can enhance the antimicrobial activity of conventional antibiotics. Industrial crops Prod. 71, 37–43. doi:10.1016/j.indcrop.2015.03.067
Bussmann, R. W., Paniagua Zambrana, N. Y., Romero, C., and Hart, R. E. (2018). Astonishing diversity-the medicinal plant markets of Bogotá, Colombia. J. Ethnobiol. Ethnomed. 14 (1), 43. doi:10.1186/s13002-018-0241-8
Chekem, M. S. G., Lunga, P. K., Tamokou, J. d. D., Kuiate, J. R., Tane, P., Vilarem, G., et al. (2010). Antifungal properties of Chenopodium ambrosioides essential oil against candida species. Pharmaceuticals 3 (9), 2900–2909. doi:10.3390/ph3092900.deCarvalho
de Carvalho, A. A., Bertolucci, S. K. V., da Silva, G. M., da Cunha, S. H. B., Roza, H. L. H., Aazza, S., et al. (2018a). Mesos components (CaCl2, MgSO4, KH2PO4) induced changes in growth and ascaridole content of Dysphania ambrosioides L. in vitro. Industrial Crops Prod. 122, 28–36. doi:10.1016/j.indcrop.2018.05.042
de Carvalho, A. A., Bertolucci, S. K. V., Honorato, A. d. C., Rocha, T. T., Silva, S. T., and Pinto, J. E. B. P. (2020). Influence of light spectra and elicitors on growth and ascaridole content using in vitro cultures of Dysphania ambrosioides L. Plant Cell Tiss. Organ Cult. 143 (2), 277–290. doi:10.1007/s11240-020-01892-5
de Carvalho, A. A., Bertolucci, S. K. V., Silva, S. T., and Pinto, J. E. B. P. (2018b). Growth and volatiles in the micropropagation of Santa Maria herb. Rev. Cienc. Agron. 49 (4), 624–635. doi:10.5935/1806-6690.20180071
de Morais Oliveira-Tintino, C. D., Tintino, S. R., Limaverde, P. W., Figueredo, F. G., Campina, F. F., da Cunha, F. A., et al. (2018). Inhibition of the essential oil from Chenopodium ambrosioides L. and α-terpinene on the NorA efflux-pump of Staphylococcus aureus. Food Chem. 262, 72–77. doi:10.1016/j.foodchem.2018.04.040
Dembitsky, V., Shkrob, I., and Hanus, L. O. (2008). Ascaridole and related peroxides from the genus Chenopodium. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech. Repub. 152 (2), 209–215. doi:10.5507/bp.2008.032
Derraji, S., Mahassin, F., Rhalem, N., and Ouzzif, Z. (2014). Hépatotoxicité par Chenopodium ambrosioides à propos de 3 observations (colligées à l'hôpital militaire d'instruction Mohammed V, Rabat - maroc). Toxicol. Anal. Clinique 26 (3), 176–180. doi:10.1016/j.toxac.2014.05.001
Foroughi, A., Pournaghi, P., Najafi, F., Zangeneh, M. M., Zangeneh, A., and Moradi, R. (2016). Chemical composition and antibacterial properties of Chenopodium botrys L. essential oil. Int. J. Pharmacogn. Phytochemical Res. 8 (11), 1881–1885.
Group, A. P. (2009). An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: Apg III. Botanical J. Linn. Soc. 161 (2), 105–121. doi:10.1111/j.1095-8339.2009.00996.x
Gupta, D., Charles, R., Mehta, V., Garg, S., and Kumar, S. (2002). Chemical examination of the essential oil of Chenopodium ambrosioides L. from the southern hills of India. J. Essent. Oil Res. 14 (2), 93–94. doi:10.1080/10412905.2002.9699780
Guyton, W. L. (1946). Poisoning due to oil of chenopodium. J. Am. Med. Assoc. 132 (6), 330–331. doi:10.1001/jama.1946.02870410018006a
Hallala, A., Benalia, S., Markouk, M., Bekkouchea, K., Larhsinia, M., Chaitb, A., et al. (2010). Evaluation of the analgesic and antipyretic activities of Chenopodium ambrosioides L. Asian J. Exp. Biol. Sci. 1 (4), 894–897.
Hassan, M., Kjos, M., Nes, I., Diep, D., and Lotfipour, F. (2012). Natural antimicrobial peptides from bacteria: Characteristics and potential applications to fight against antibiotic resistance. J. Appl. Microbiol. 113 (4), 723–736. doi:10.1111/j.1365-2672.2012.05338.x
Ignacchiti, M. D. C., de Queiroz, V. T., Martins, I. V. F., Crico, K. B., Gonçalves, L. V., Fazolo, M. B., et al. (2022). Chemical composition and effect of Dysphania ambrosioides (L.) mosyakin & clemants essential oil on Biomphalaria tenagophila (D’Orbigny, 1835). Nat. Prod. Res. 36 (10), 2595–2598. doi:10.1080/14786419.2021.1910261
Judd, W. S., and Ferguson, I. (1999). The genera of Chenopodiaceae in the southeastern United States. Harv. Pap. Bot. 4 (2), 365–416.
Kandsi, F., Elbouzidi, A., Lafdil, F. Z., Meskali, N., Azghar, A., Addi, M., et al. (2022). Antibacterial and antioxidant activity of Dysphania ambrosioides (L.) mosyakin and clemants essential oils: Experimental and computational approaches. Antibiotics 11 (4), 482. doi:10.3390/antibiotics11040482
Kasali, F. M., Tusiimire, J., Kadima, J. N., and Agaba, A. G. (2021). Ethnomedical uses, chemical constituents, and evidence-based pharmacological properties of Chenopodium ambrosioides L.: Extensive overview. Futur. J. Pharm. Sci. 7 (1), 1–36. doi:10.1186/s43094-021-00306-3
Khan, M. N., and Jan, A. (2019). Evaluation of pharmacognostic features and antimicrobial activities of Dysphania botrys L. Sarhad J. Agric. 35 (4), 1234–1242. doi:10.17582/journal.sja/2019/35.4.1234.1242
Kim, Y., Park, J., and Chung, Y. (2019). Comparative analysis of chloroplast genome of Dysphania ambrosioides (L.) Mosyakin & Clemants understanding phylogenetic relationship in genus Dysphania R. Br. Korean J. Plant Resour. 32 (6), 644–668. doi:10.7732/kjpr.2019.32.6.644
Kokanova-Nedialkova, Z., Nedialkov, P. T., and Nikolov, S. D. (2009). The genus Chenopodium: Phytochemistry, ethnopharmacology and pharmacology. Pharmacogn. Rev. 3 (6), 280–306.
Kola-Mustapha, A. T., Yohanna, K. A., Ghazali, Y. O., and Ayotunde, H. T. (2020). Design, formulation and evaluation of Chasmanthera dependens Hochst and Chenopodium ambrosioides Linn based gel for its analgesic and anti-inflammatory activities. Heliyon 6 (9), e04894. doi:10.1016/j.heliyon.2020.e04894
Li, J., Yang, X., Yu, J., Li, Z., Deng, Q., Cao, Y., et al. (2020). Chemical composition of the volatile oil of Chenopodium ambrosioides L. from Mianyang in Sichuan Province of China and its sub-chronic toxicity in mice. Trop. J. Pharm. Res. 19 (9), 1985–1991. doi:10.4314/tjpr.v19i9.26
Limaverde, P. W., Campina, F. F., da Cunha, F. A., Crispim, F. D., Figueredo, F. G., Lima, L. F., et al. (2017). Inhibition of the TetK efflux-pump by the essential oil of Chenopodium ambrosioides L. and α-terpinene against Staphylococcus aureus IS-58. Food Chem. Toxicol. 109, 957–961. doi:10.1016/j.fct.2017.02.031
Łuczaj, Ł., Wolanin, M., Drobnik, J., Kujawska, M., Dumanowski, J., Walker, K., et al. (2022). Dysphania schraderiana (Schult.) Mosyakin & Clemants–An overlooked medicinal and ritual plant used in Poland. J. Ethnopharmacol. 284, 114755. doi:10.1016/j.jep.2021.114755
Machín, L., Tamargo, B., Piñón, A., Atíes, R. C., Scull, R., Setzer, W. N., et al. (2019). Bixa orellana L.(Bixaceae) and Dysphania ambrosioides (L.) Mosyakin & Clemants (Amaranthaceae) essential oils formulated in nanocochleates against Leishmania amazonensis. Molecules 24 (23), 4222. doi:10.3390/molecules24234222
Mahboubi, M., Bidgoli, F. G., and Farzin, N. (2011). Chemical composition and antimicrobial activity of Chenopodium botrys L. essential oil. J. Essent. Oil Bear. Plants 14 (4), 498–503. doi:10.1080/0972060x.2011.10643608
Mokni, R. E., Youssef, F. S., Jmii, H., Khmiri, A., Bouazzi, S., Jlassi, I., et al. (2019). The essential oil of Tunisian Dysphania ambrosioides and its antimicrobial and antiviral properties. J. Essent. Oil Bear. Plants 22 (1), 282–294. doi:10.1080/0972060X.2019.1588171
Monzote, L., García, M., Pastor, J., Gil, L., Scull, R., Maes, L., et al. (2014). Essential oil from Chenopodium ambrosioides and main components: Activity against Leishmania, their mitochondria and other microorganisms. Exp. Parasitol. 136 (1), 20–26. doi:10.1016/j.exppara.2013.10.007
Monzote, L., Geroldinger, G., Tonner, M., Scull, R., De Sarkar, S., Bergmann, S., et al. (2018). Interaction of ascaridole, carvacrol, and caryophyllene oxide from essential oil of Chenopodium ambrosioides L. with mitochondria in Leishmania and other eukaryotes. Phytother. Res. 32 (9), 1729–1740. doi:10.1002/ptr.6097
Morteza-Semnani, K.Department of Medicinal Chemistry, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran (2015). A review on Chenopodium botrys L.: Traditional uses, chemical composition and biological activities. Mazums-pbr. 1 (2), 1–9. doi:10.18869/acadpub.pbr.1.2.1
Mosa, K. A., Ismail, A., and Helmy, M. (2017). “Introduction to plant stresses,” in Plant stress tolerance (Springer), 1–19.
Mosyakin, S., and Clemants, S. (2002). New nomenclatural combinations in Dysphania R. Br. (Chenopodiaceae): Taxa occurring in north America. Ukr. Bot. Zhurnal 59, 380–385.
Mroczek, A. (2015). Phytochemistry and bioactivity of triterpene saponins from Amaranthaceae family. Phytochem. Rev. 14 (4), 577–605. doi:10.1007/s11101-015-9394-4
Murray, P. M., Moane, S., Collins, C., Beletskaya, T., Thomas, O. P., Duarte, A. W., et al. (2013). Sustainable production of biologically active molecules of marine based origin. N. Biotechnol. 30 (6), 839–850. doi:10.1016/j.nbt.2013.03.006
Nagle, D., Mahdi, F., Datta, S., Li, J., Du, L., Smillie, T., et al. (2011). Assessing the potential mitochondrial-mediated toxicity of herbal dietary supplements. Planta Med. 77 (05), S11. doi:10.1055/s-0031-1273513
Ndhlala, A. R., Ncube, B., Okem, A., Mulaudzi, R. B., and Van Staden, J. (2013). Toxicology of some important medicinal plants in southern Africa. Food Chem. Toxicol. 62, 609–621. doi:10.1016/j.fct.2013.09.027
Paniagua-Zambrana, N. Y., Bussmann, R. W., and Romero, C. (2020). Dysphania ambrosioides (L.) Mosyakin & Clemants Amaranthaceae, 1–9. doi:10.1007/978-3-319-77093-2_106-1
Pereira-de-Morais, L., de Alencar Silva, A., da Silva, R. E. R., Navarro, D. M. d. A. F., Coutinho, H. D. M., de Menezes, I. R. A., et al. (2020). Myorelaxant action of the Dysphania ambrosioides (L.) Mosyakin & Clemants essential oil and its major constituent α-terpinene in isolated rat trachea. Food Chem. 325, 126923. doi:10.1016/j.foodchem.2020.126923
Pino, J. A., Marbot, R., and Real, I. M. (2003). Essential oil of Chenopodium ambrosioides L. from Cuba. J. Essent. Oil Res. 15 (3), 213–214. doi:10.1080/10412905.2003.9712118
Potawale, S. E., Luniya, K. P., Mantri, R. A., Mehta, U. K., Sadiq, M. D., Waseem, M. D., et al. (2008). Chenopodium ambrosioides: An ethnopharmacological review. Pharmacologyonline 2, 272–286.
Prasad, C. S., Shukla, R., Kumar, A., and Dubey, N. (2010). In vitro and in vivo antifungal activity of essential oils of Cymbopogon martini and Chenopodium ambrosioides and their synergism against dermatophytes. Mycoses 53 (2), 123–129. doi:10.1111/j.1439-0507.2008.01676.x
Sá, R. D., Santana, A. S. C. O., Silva, F. C. L., Soaresa, L. A. L., and Randaua, K. P. (2016). Anatomical and histochemical analysis of Dysphania ambrosioides supported by light and electron microscopy. Rev. Bras. Farmacogn. 26 (5), 533–543. doi:10.1016/j.bjp.2016.05.010
Santiago, J. A., Cardoso, M. D. G., Batista, L. R., Castro, E. M. d., Teixeira, M. L., and Pires, M. F. (2016). Essential oil from Chenopodium ambrosioides L.: Secretory structures, antibacterial and antioxidant activities. Acta Sci. Biol. Sci. 38 (2), 139. doi:10.4025/actascibiolsci.v38i2.28303
Sayyedrostami, T., Pournaghi, P., Vosta-Kalaee, S. E., and Zangeneh, M. M. (2018). Evaluation of the wound healing activity of Chenopodium botrys leaves essential oil in rats (a short-term study). J. Essent. Oil Bear. Plants 21 (1), 164–174. doi:10.1080/0972060x.2018.1451394
Shameem, S. A., Khan, K. Z., Waza, A. A., Shah, A. H., Qadri, H., and Ganai, B. A. (2019). Bioactivities and chemoprofiling comparisons of Chenopodium ambrosioides L and Chenopodium botrys L. growing in Kashmir India. Asian J. Pharm. Clin. Res. 12 (1), 124–129. doi:10.22159/ajpcr.2019.v12i1.28418
Sin, J., Mangale, V., Thienphrapa, W., Gottlieb, R. A., and Feuer, R. (2015). Recent progress in understanding coxsackievirus replication, dissemination, and pathogenesis. Virology 484, 288–304. doi:10.1016/j.virol.2015.06.006
Singh, H., Batish, D., Kohli, R., Mittal, S., and Yadav, S. (2008). Chemical composition of essential oil from leaves of Chenopodium ambrosioides from Chandigarh, India. Chem. Nat. Compd. 44 (3), 378–379. doi:10.1007/s10600-008-9070-7
Singh, P., and Pandey, A. K. (2021). Dysphania ambrosioides essential oils: From pharmacological agents to uses in modern crop protection—a review. Phytochem. Rev. 21, 141–159. doi:10.1007/s11101-021-09752-6
Sukhorukov, A. P., Kushunina, M., and Verloove, F. (2016). Notes on Atriplex, oxybasis and Dysphania (Chenopodiaceae) in west-central tropical africa. Plecevo. 149 (2), 249–256. doi:10.5091/plecevo.2016.1181
The Plant List (2020). The plant list. Available: http://www.theplantlist.org/([Accessed].
Tzakou, O., Pizzimenti, A., Pizzimenti, F., Sdrafkakis, V., and Galati, E. (2007). Composition and antimicrobial activity of Chenopodium botrys L. Essential oil from Greece. J. Essent. Oil Res. 19 (3), 292–294. doi:10.1080/10412905.2007.9699284
Uotila, P., Sukhorukov, A. P., Bobon, N., McDonald, J., Krinitsina, A. A., and Kadereit, G. (2021). Phylogeny, biogeography and systematics of dysphanieae (Amaranthaceae). Taxon 70 (3), 526–551. doi:10.1002/tax.12458
Verma, N., and Shukla, S. (2015). Impact of various factors responsible for fluctuation in plant secondary metabolites. J. Appl. Res. Med. Aromatic Plants 2 (4), 105–113. doi:10.1016/j.jarmap.2015.09.002
Villalobos-Delgado, L. H., González-Mondragón, E. G., Ramírez-Andrade, J., Salazar-Govea, A. Y., and Santiago-Castro, J. T. (2020). Oxidative stability in raw, cooked, and frozen ground beef using Epazote (Chenopodium ambrosioides L.). Meat Sci. 168, 108187. doi:10.1016/j.meatsci.2020.108187
Wei, H., Liu, J., Li, B., Zhan, Z., Chen, Y., Tian, H., et al. (2015). The toxicity and physiological effect of essential oil from Chenopodium ambrosioides against the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Crop Prot. 76, 68–74. doi:10.1016/j.cropro.2015.06.013
Ya-Nan, W., Jia-Liang, W., Dan-Wei, M., Jiao, L., and Du-Yu, Z. (2015). Anticancer effects of Chenopodium ambrosiodes L. essential oil on human breast cancer MCF-7 cells in vitro. Trop. J. Pharm. Res. 14 (10), 1813–1820. doi:10.4314/tjpr.v14i10.11
Yabuta, Y., Mieda, T., Rapolu, M., Nakamura, A., Motoki, T., Maruta, T., et al. (2007). Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. J. Exp. Bot. 58 (10), 2661–2671. doi:10.1093/jxb/erm124
Ye, H., Liu, Y., Li, N., Yu, J., Cheng, H., Li, J., et al. (2015). Anti-Helicobacter pylori activities of Chenopodium ambrosioides L. in vitro and in vivo. World J. Gastroenterol. 21 (14), 4178–4183. doi:10.3748/wjg.v21.i14.4178
Yossen, M. B., Lozada, M., Kuperman, M. N., González, S., Gastaldi, B., and Buteler, M. (2019). Essential oils as vespid wasp repellents: Implications for their use as a management strategy. J. Appl. Entomol. 143 (6), 635–643. doi:10.1111/jen.12631
Yousefi, N., Chehregani, A., Malayeri, B., Lorestani, B., and Cheraghi, M. (2011). Investigating the effect of heavy metals on developmental stages of anther and pollen in Chenopodium botrys L. (Chenopodiaceae). Biol. Trace Elem. Res. 140 (3), 368–376.
Keywords: Dysphania, ethnophamacology, essential oils, medicinal benefits, toxicology
Citation: Dagni A, Hegheș SC, Suharoschi R, Pop OL, Fodor A, Vulturar R, Cozma A, Aniq filali O, Vodnar DC, Soukri A and El Khalfi B (2022) Essential oils from Dysphania genus: Traditional uses, chemical composition, toxicology, and health benefits. Front. Pharmacol. 13:1024274. doi: 10.3389/fphar.2022.1024274
Received: 21 August 2022; Accepted: 21 November 2022;
Published: 08 December 2022.
Edited by:
Daniela Rigano, University of Naples Federico II, ItalyReviewed by:
Fatma Moharram, Helwan University, EgyptAmner Muñoz-Acevedo, Universidad del Norte, Colombia
Copyright © 2022 Dagni, Hegheș, Suharoschi, Pop, Fodor, Vulturar, Cozma, Aniq filali, Vodnar, Soukri and El Khalfi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Oana Lelia Pop, oana.pop@usamvcluj.ro; Ramona Suharoschi, ramona.suharoschi@usamvcluj.ro; Bouchra El Khalfi, bouchra.elkhalfi@gmail.com
†These authors have contributed equally to this work and share first authorship
†These authors have contributed equally to this work and share last authorship
 Amal Dagni
Amal Dagni Simona Codruta Hegheș
Simona Codruta Hegheș Ramona Suharoschi
Ramona Suharoschi Oana Lelia Pop
Oana Lelia Pop Adriana Fodor
Adriana Fodor Romana Vulturar
Romana Vulturar Angela Cozma
Angela Cozma Oufaa Aniq filali
Oufaa Aniq filali Dan Cristian Vodnar
Dan Cristian Vodnar Abdelaziz Soukri1
Abdelaziz Soukri1