- 1School of Pharmacy, ITM University, Gwalior, Gwalior, Madhya Pradesh, India
- 2School of Studies in Pharmaceutical Sciences, Jiwaji University, Gwalior, Madhya Pradesh, India
- 3School of Sciences, ITM University, Gwalior, Gwalior, Madhya Pradesh, India
- 4Faculty of Pharmacy, Philadelphia University, Amman, Jordan
- 5Department of Pharmacology, College of Pharmacy, King Khalid University, Abha, Saudi Arabia
- 6Department of Microbiology and Clinical Parasitology, College of Medicine, King Khalid University, Abha, Saudi Arabia
- 7Center for Stem Cell Research, College of Medicine, King Khalid University, Abha, Saudi Arabia
- 8Department of Anatomy, College of Medicine, King Khalid University, Abha, Saudi Arabia
- 9Department of Pharmacy, Pranveer Singh Institute of Technology, Kanpur, India
- 10College of Pharmacy and Nursing, University of Nizwa, Nizwa, Oman
Ethnopharmacological relevance: Alchornea laxiflora (Benth.) Pax & K. Hoffm. (Euphorbiaceae) is an important traditional medicinal plant grown in tropical Africa. The stem, leaves, and root have been widely used in the folk medicine systems in Nigeria, Cameroon, South Africa, and Ghana to treat various ailments, including inflammatory, infectious, and central nervous system disorders, such as anxiety and epilepsy.
Material and methods: The scientific name of the plant was validated using the “The Plant List,” “Kew Royal Botanic Gardens,” and Tropicos Nomenclatural databases. The literature search on A. laxiflora was performed using electronic search engines and databases such as Google scholar, ScienceDirect, PubMed, AJOL, Scopus, and Mendeley.
Results: To the best of our knowledge, no specific and detailed review has been reported on A. laxiflora. Consequently, this review provides an up-to-date systematic presentation on ethnobotany, phytoconstituents, pharmacological activities, and toxicity profiles of A. laxiflora. Phytochemical investigations disclosed the presence of important compounds, such as alkaloids, flavonoids, phenolics, terpenoids, and fatty acids. Furthermore, various pharmacological activities and traditional uses reported for this botanical drug were discussed comprehensively.
Conclusion: This systemic review presents the current status and perspectives of A. laxiflora as a potential therapeutic modality that would assist future researchers in exploring this African botanical drug as a source of novel drug candidates for varied diseases.
Introduction
Indigenous herbal medicines are the first-line treatment for most third-world countries (Mahomoodally, 2013). According to the World Health Organization (WHO), about 80% of the world population employs herbal medicine for their primary health care using plant extracts (Mahomoodally, 2013; Nabatanzi et al., 2020). Various factors encourage herbal medicines, such as acceptability, poverty, cost-effectiveness, accessibility, and unavailability of modern health facilities (Hossain et al., 2014). However, there are concerns about the toxic effects of certain botanical drugs if used unchecked and irrationally (Firenzuoli and Gori, 2007; Okaiyeto and Oguntibeju, 2021). Globally, 28,187 plant species have been recorded as constituting medicinal use in 416 families of angiosperm plants. Euphorbiaceae is among the top three families with a significantly higher proportion of medicinal plants (Phumthum et al., 2019).
The Euphorbiaceae or spurge family comprises monoecious/dioecious herbs, shrubs, vines, or trees. Major groups of this family contain latex and have cosmopolitan distribution (Agbo et al., 2020). Economically important members include the natural rubber plant (Hevea brasiliensis (Willd. ex A. Juss.) Müll. Arg.), Tapioca plant (Manihot esculenta Crantz), castor oil (Ricinus communis L.), tung oil (Vernicia fordii (Hemsl.) Airy Shaw), candlenut oil (Aleurites moluccanus (L.) Willd.) and various oil, timber, medicinal, dye, and ornamental plants (Simpson, 2010; Agbo et al., 2020).
According to “The Plant List” (http://www.theplantlist.org), the Alchornea genus consists of 55 species. It has pan-tropical distribution with a strong tendency to tropical rain forests in American, African, and Asian countries. Alchornea laxiflora (Benth.) Pax & K. Hoffm. (A. laxiflora) is one of the accepted species of the Alchornea genus (Martínez et al., 2017).
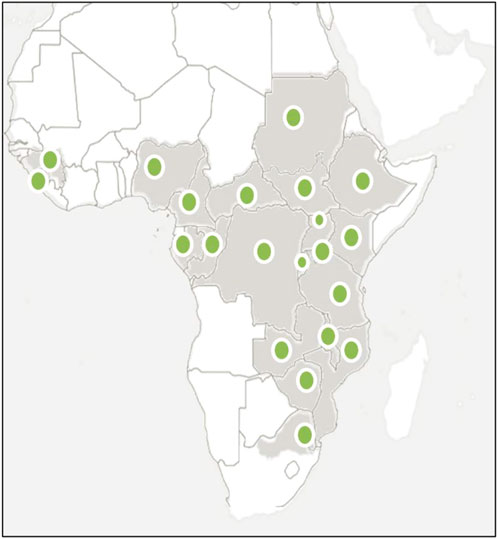
A. laxiflora is endemic to Africa and is widely distributed (Figure 1) in central, eastern, and southern tropical African countries, namely, Burundi, Central African Republic, Congo, Ethiopia, Gabon, Guinea, Gulf of Guinea, Kenya, Malawi, Mozambique, Northern Provinces, Rwanda, Sierra Leone, Sudan, Swaziland, Tanzania, Uganda, Zambia, Zaïre, and Zimbabwe (Höft and Höft, 1995; Muller et al., 2005; Mwavu and Witkowski, 2008; Obodai and Nsor, 2009; Essiett and Ajibesin, 2010; Molander et al., 2014; McCarthy et al., 2017; Tegene, 2018; Verlhac et al., 2018; Magwede et al., 2019; Siwe-Noundou et al., 2019).
A. laxiflora grows best from the sea level up to 1,600 m altitude and is widely spread in evergreen forests, associated bushland in fire-protected places, and deciduous and riverine thickets near coasts (Burkill, 1985; Aweto, 2001; Obodai and Nsor, 2009). A. laxiflora primarily has four synonyms: Alchornea engleri Pax, Alchornea schlechteri Pax, Lepidoturus laxiflorus Benth., and Macaranga thonneri De Wild. The common names of A. laxiflora are “Lowveld bead string/Venda bead string/three veined bead string,” derived from the shape of its open inflorescences. It has several vernacular names depending on the cultural and ethnic diversity in Africa (Oladunmoye and Kehinde, 2011; Gbadamosi, 2015; Magwede et al., 2019). In the Ekiti state of Nigeria, it is also known as Canestiks and Arithmetic stick (Adeniran, 2015; Olanipekun and Aladetimiro (2017)). Some local African names are Uwenuwen, Ukpo-ubieka, Uwenriotan (Edo), Ububo (Igbo), Ijan, Ijun, Ijan furfur, Ijàndú, Igiiya, Pepe, Ewe lya, Ewe pepe, iyapepe, Opoto, Gbogbonse (Yoruba), Nwariwa (Ibibio), Urievwu, Urie vivu (Urhobo), Fura amarya (Hausa), Vendakralesnoer (Afrikaans), mubvamalofha and murundamalofha (Tshivenḓa), Murarahomba and Muruka (Shona), Eholo (Bakossi), Nnami (Kimwera), Mechango (Bambalang), Josos (Bakweri), Meshé (Bamoun), and Akwukwo Ugba (Njamen et al., 2013; Bafor et al., 2018, 2015; Akinpelu et al., 2015; Gbadamosi, 2015; Okokon et al., 2017b; Nwonu et al., 2018a; Bamimore and Elujoba, 2018; Magwede et al., 2019; Oluyemi and Blessing, 2019).
Recently, it has been established that medicinal plants are rich sources for new drug development, and traditional medicinal data has a quite good success rate in new therapeutics (Ochieng et al., 2022). Africa represents about a quarter of the world trade of biodiversity, and it is surprising that only a few drugs have been commercialized compared to other countries (Maroyi, 2016; Ali et al., 2017; Nabatanzi et al., 2020). The reason could be the lack of documentation, the secretive practices of local healers and folklore medicine practitioners, or lack of interest by first-world countries (Geethangili and Ding, 2018). A. laxiflora is one of the least explored plants possessing diverse ethnomedicinal and non-medicinal uses, as reported from different cultures and localities in Africa for centuries. However, it has gained the scientific interest of researchers in the last 2 decades regarding its pharmacological activities. A few studies have been conducted to identify and isolate the bio-constituents, and only limited reports are available for pharmacological studies. Although this plant has a wide distribution throughout Africa, it is worth noting that only Nigeria and Cameroon were the countries with the highest number of reports considering plant occurrence, traditional uses, and pharmacological activities.
A couple of reviews broadly summarized the traditional and pharmacological uses of the Alchornea genus, primarily from Alchornea cordifolia (Schumach. & Thonn.) Müll. Arg. and Alchornea floribunda Müll. Arg. (Boniface et al., 2016; Agbo et al., 2020). However, no specific and detailed review has been reported in the literature on A. laxiflora. This review is intended to present detailed information systematically on the ethnobotany, phytochemistry, and pharmacology of A. laxiflora. Furthermore, this review will explore the therapeutic potential and evaluate future research opportunities pertaining to A. laxiflora. Figure 2 summarizes the crucial information on A. laxiflora.
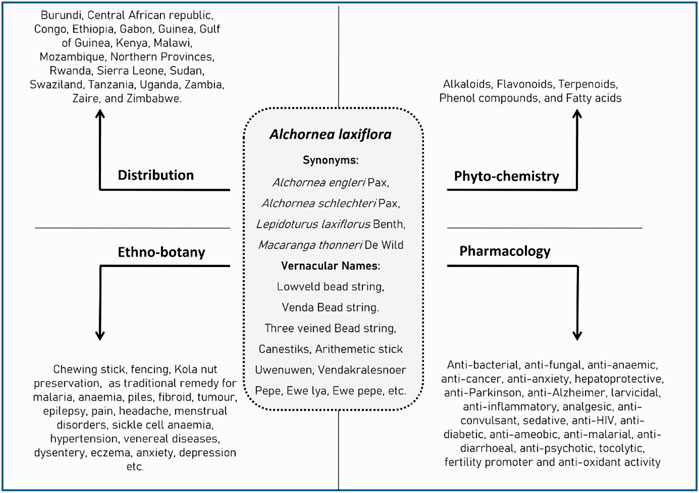
FIGURE 2. Summary of synonyms, geographical distribution, phytochemistry, ethnobotany, and pharmacology of A. laxiflora.
Methods
The literature search was done from various search engines and databases such as PubMed, Google Scholar, ScienceDirect, AJOL, Scopus, and Mendeley. We examined the literature published before June 2021 on ethnomedicinal uses, phytochemistry, pharmacology of extracts, and isolated compounds of A. laxiflora. Following the general guidelines on scientific nomenclature for plants to avoid any ambiguity and errors (Rivera et al., 2014), the species names, families, plant authority, and synonyms were verified using books, journal articles, and Webpages such as the “Kew Royal Botanic Gardens” (mpns.kew.org), the Missouri Botanical Garden’s Tropicos Nomenclatural database (www.tropicos.org), and “The Plant List” (www.theplantlist.org). The search terms “Alchornea laxiflora” or “A. laxiflora extract” or “A. laxiflora compounds” were used with no specified time limit. All articles with potential full-texts and titles/abstracts were included, and no language restrictions were applied. All the relevant references were checked for additional and unpublished citations. Most ethnobotanical data were collected from Nigeria, Cameroon, South Africa, and Zimbabwe. The pharmacological research literature on A. laxiflora was critically assessed for the general requirements of the pharmacological research of botanical drugs as suggested by Heinrich et al. (2020), and only the literature that met the general requirements was considered in this presented review.
Botanical description and distribution
A. laxiflora is a deciduous understorey tree or shrub that grows up to 6 m tall and is often found in places such as lowland tropical forests, wetlands, riverine vegetation, mixed deciduous woodlands, sub-montane forests, and semi-deciduous tropical rainforests (Mwavu and Witkowski, 2008; Obodai and Nsor, 2009; Tegene, 2018; Verlhac et al., 2018). The leaves are simple and alternate in arrangement, elliptic-lanceolate to oblong-lanceolate in shape, with dimensions of up to 17 × 8 cm. Moreover, the leaves are thinly structured, light green in color, turning to yellow, or red color in the dry season with three-veined venation from the base and shallowly crenate-serrate margination (Figure 3). The young leaves appear purple in color. The plant is monoecious, with male and female inflorescence on separate branches (Akinpelu et al., 2015). The flowers are unisexual on the same plant with conspicuous reddish bracts. The fruit is 5–7 mm in diameter with two-to-four-lobed capsules, which are thinly woody and blackish-brown (Hutchinson and Dalziel, 1937; Burkill, 1985). This plant has multiple traditional uses, but no attempts have been made to domesticate it. It was found to be key in re-sprouting woody species in natural or manmade disturbed forests (Mwavu and Witkowski, 2008). In Uganda, A. laxiflora was reported to be one of the several novel plant species used by Chimpanzees to make nests under anthropogenic pressure of habitat loss (McCarthy et al., 2017).
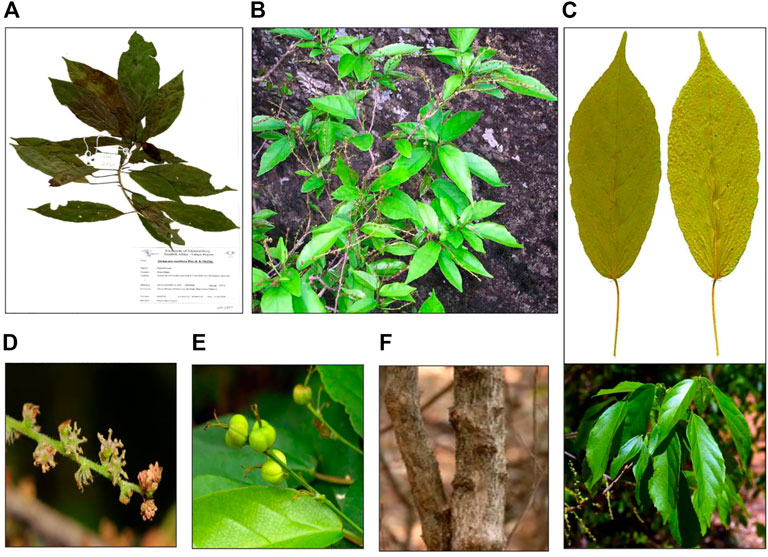
FIGURE 3. Morphology of A. laxiflora: herbarium specimen (A), whole plant (B), leaves (C), inflorescence (D), fruits (E), and stem(F) (https://www.zimbabweflora.co.zw/).
Ethnobotany
Traditionally, all parts of A. laxiflora have utility in folk medicine for various purposes in different regions of Africa. Interestingly, maximum reports of traditional uses were from Nigeria and Cameroon, DR Congo, South Africa, and Ghana. However, no literature reports are noticed from Tanzania, Kenya, Kenya, Malawi, Mozambique, Northern Provinces, Rwanda, Sierra Leone, Sudan, Swaziland, Tanzania, Uganda, Zambia, Zaïre, and Zimbabwe. Other than medicinal uses, A. laxiflora has deep-rooted effects on the environmental and cultural aspects of Africa. A diversity of folk medicine applications of A. laxiflora are emmenagogue, promoting dental hygiene, easing toothache, and managing sickle cell diseases, as well as being anti-diabetic, anti-inflammatory, antioxidant, anti-infectious, anti-anemic, antifungal, and hepato-protective (Figure 2).
Whole plant
In the South and Southwestern regions of Nigeria, this woody plant is an important component of rural architecture. Natives used to construct life fences (Fongod et al., 2014). In South Africa and DR Congo, A. laxiflora is reported to be used as an anti-venom for snake bites (Molander et al., 2014). Similarly, the whole plant has been reported for the treatment of malaria, pile, dysentery, eczema, cough, and high fever in the Ekiti state of Nigeria (Jayeoba et al., 2012; Adeniran, 2015).
Leaves
Leaves are the most frequently used plant part in folk medicine preparations, taken orally in most instances as an infusion, decoction, or juice, followed by the stems, branches, and roots. Leaves crushed in water are sometimes applied externally to treat skin diseases (Ajibesin et al., 2007). Similarly, the leaves of A. laxiflora are squeezed and mixed with milk or cheese (wara). A cup of the resultant mixture is suggested to be taken twice a week as a remedy for anemia by the Yoruba tribe of southwest Nigeria (Fabeku and Akinsulire, 2008). For managing uterine contraction and prevention of miscarriages, traditional healers in the south of Nigeria use A. laxiflora leave concoction prepared by squeezing leaves in water, filtering them, and mixing them with calabash chalk or calabash clay, taken twice a day after conception for 6 months (Bafor et al., 2018). Moreover, in the food industry, A. laxiflora leaves are used as wraps to preserve Kola nuts (Garcinia kola) and other perishable food items (Ihejirika et al., 2015).
Stem (bark and branchlets)
The stem branches are used as Chewing sticks (local toothbrushes) for cleaning teeth in Nigeria (Farombi et al., 2003). Stem bark and branches have also been used in traditional medicine for various purposes, notably for malaria, anemia, emmenagogue, ringworm, venereal disease, typhoid fever, antioxidant, infertility in females, infectious diseases, tumor, inflammation, teething problems, and toothache in South Africa, Ghana, and Nigeria (Dzoyem and Eloff, 2015; Kaur et al., (2012); Obodai and Nsor, 2009).
Root
In the Ogun and Osun states of Nigeria, the roots and fruits are used as ethnomedicine to treat fibroids (Oyeyemi et al., 2019). In the Edo state of Nigeria, the root bark of A. laxiflora is boiled with an egg from local chicken and eaten as a remedy for hemorrhoids (Ugbogu and Chukwuma, 2019). The traditional practitioners in the Ekiti state of Nigeria also prescribe root decoction for lowering blood pressure and reducing incidences of heart failure (Adeniran and Falemu, 2017).
Traditional use of A. laxiflora is limited to Africa, with lots of similarities in therapeutic applications, possibly due to the shared cultural exchange of its ethnobotanical use. Table 1 shows the ethnomedicinal uses of A. laxiflora in different African countries, regions, and communities, together with the plant parts used.
Phytochemistry
Although various phytochemical screening studies have suggested the presence of multiple classes of chemical constituents such as alkaloids, saponins, tannins, flavonoids, phenols, steroids, cardio-active glycosides, and reducing sugars, more studies are required to fully characterize the phytochemistry. However, the flowers and fruits of this plant species have not been studied extensively to identify phytoconstituents, relating to their low degree of non-usage in traditional medicines. The leaves, stems, and barks are highly exploited parts of the plant for the isolation and identification of phytoconstituents. The leaf has a higher diversity of phytochemicals compared to other plant parts. All the isolated compounds from A. laxiflora are mentioned in Table 2, and their chemical structures are presented in Supplementary Figures S1–S7.
Flavonoids
Flavonoids are the most common group of natural polyphenolic substances found in all fruits and vegetables. The flavonoids reported in A. laxiflora are in the form of flavanol and glycoside. From the ethyl acetate soluble fraction of the crude methanolic leaf extract of A. laxiflora, one novel acetylated flavonoid quercetin-3,4′-diacetate and three known flavonoid glycosides, quercetin, quercitrin, and rutin, were isolated (Ogundipe et al., 2001b). Concurrently, two novel sulfated flavonoids were isolated for the first time in the genus Alchornea and the family Euphorbiaceae, namely, quercetin-7,4′-disulphate and quercetin-3′,4′-disulphate (Ogundipe et al., 2001a). In another study, taxifolin glycosides were isolated from an n-butanol fraction of crude 50% ethanol aqueous leaf extract by AGC and SLHC chromatography characterized by spectroscopy techniques such as MS, 1H, and 13C NMR (Adeloye et al., 2005). Oloyede et al. (2011) reported that A. laxiflora leaves’ ethanol extract on fractionation and characterization yielded two new flavonoids, namely, quercetin-3-O-β-D-glucopyranoside and quercetin-3,7.3′,4′-tetrasulphate. Lately, five known flavonoid glycosides (hyperoside, reynoutrin, guaijaverin, taxifolin-3-O-β-D-xylopyranoside, taxifolin-3-O-β-D-galactopyranoside) and two megastigmane glycosides (byzantionoside B and leeaoside) together with one steroidal glycoside (β-sitosterol-β-D-glucoside) and one lignan glycoside (syringaresinol-β-D-glucoside) were isolated from the methanolic leaf extract of A. laxiflora (Tapondjou et al., 2016). Interestingly, flavonoids were reported mainly from the leaves, but other parts of the plant have not been investigated yet, and quercetin sounds to be the most abundant and common monomer in the plant (Table 2; Supplementary Figure S1).
Phenolic compounds
The leaves of A. laxiflora have demonstrated a higher concentration of phenolic compounds than the other parts (Table 2). The phytochemical investigation of methanolic extract from the stem bark of A. laxiflora resulted in the isolation of eight compounds, including ellagic acid, 3-O-methylellagic acid, and 3-O-methylellagic acid-3-O-α-rhamnopyranoside (Sandjo et al., 2011). A novel ellagic acid derivative, namely, 3,4,3′-tri-O-methylellagic acid, was isolated from the methanolic extract obtained from the A. laxiflora stem bark (Mbaveng et al., 2015). In another study, Morah and Uduagwu (2017) separated several phenolic compounds, namely, phenol, 2,4-bis(1,1-dimethylethyl)-; phenol, 2,6-bis(1,1-dimethylethyl)-; phthalic acid, butyl undecyl ester; diisooctyl phthalate and bis[di(trimethylsiloxy) phenylsiloxy] trimethylsiloxy phenyl siloxane from the petroleum ether; and ethanol extract of A. laxiflora leaves. In addition, Okokon et al. (2017b) isolated butylated hydroxy anisole (BHA), pyrogallol, 1,2-benzenedicarboxylic acid; 4-((1E)-3-hydroxy-1-propenyl)-2-methoxyphenol; 2-methoxy-4-vinylphenol; and 4-vinylphenol as major constituents of A. laxiflora leaves. 1,1′-Biphenyl-3,4,4′-trimethoxy-6′-formyl-; phenol, 3-[(trimethylsilyl)oxy]-; and zeranol were reported to be isolated from the A. laxiflora root ethyl acetate fraction (Okokon et al., 2017a) (Table 2; Supplementary Figure S2).
Terpenoids
Many terpenoids (22 compounds), including seven triterpenoids, two diterpenoids, one sesquiterpene, five carotenoids, and seven steroids, have been isolated from the leaves, stem bark, and root extracts of A. laxiflora (Table 2; Supplementary Figure S3). Triterpenoids are represented by four pentacyclic triterpenoids (3-acetyloleanolic acid, 3-acetoxyursolic acid, adipedatol, and betulin) and two squalene types (squalene and 2,2,4-trimethyl-3-(3,8,12,16-tetramethyl-heptadeca-3,7,11,15-tetraenyl)-cyclohexanol) triterpenoid. Diterpenoids isolated from A. laxiflora leaves’ methanol and petroleum ether extracts are 17-hydroxyingenol and 3,7,11,15-tetramethyl-2-hexadecen-1-ol (Sandjo et al., 2011; Tapondjou et al., 2016; Okokon et al., 2017b; 2017a; Morah and Uduagwu, 2017; Bafor et al., 2018). Morah and Uduagwu (2017) investigated five carotenoid pigments (astaxanthin, lycoxanthin, rhodopin, dimethoxy-lycopene, and anhydrorhodovibrin), one sesquiterpene (hexahydrofarnesyl acetone), and five steroids (glycocholic acid, ethyl iso-allocholate, 7,8-epoxylanostan-11-ol, 3-acetoxy-, and 4-vinylcholestan-3-ol) from leaves of A. laxiflora using petroleum ether and ethanol fraction and structure confirmed by GS-MS. Sandjo et al. (2011) and Tapondjou et al. (2016) isolated and established the structure of a known steroidal glycoside, β-sitosterol-3-O-β-D-glucopyranoside from the methanol extract of stem bark and leaves, respectively. Likewise, another steroid cholest-4-en-3-one was isolated and characterized by Okokon et al. (2017a) from A. laxiflora ethanol root extract. In an attempt to isolate some antimalarial and antiplasmodial constituents from A. laxiflora, leaves and root ethanol extract on fractionation resulted in the isolation of three terpenoids, namely, 2,6,10-trimethylundecan-(5E)-2,5,9-trien-4-one; 3,7,11,15-tetramethyl-2-hexadecen-1-ol and 2(4H)-benzofuranone, 5,6,7,7A-tetrahydro-6-hydroxy-4,4,7- (Okokon et al., 2017b; 2017a).
Fatty acids
Recently, essential oil from A. laxiflora leaves hydro-distillate on GC-MS analysis offered three long-chain aliphatic acids (palmitic, oleic, and petroselinic) (Otuechere et al., 2019). In another study, the crude methanolic extract of A. laxiflora leaves was subjected to column chromatographic separation and HR-ESI-TOF-MS analysis, resulting in the isolation of one novel fatty acid ester, namely, (10Z)-tetradec-10-enoic acid-(2S)-2-carboxy-2-hydroxyethyl ester, and one new ceramide, (2R)-2-hydroxy-N-[(2S,3S,4R,15Z)-1,3,4-trihydroxy-15-triaconten-2-yl]octacosamide (Sandjo et al., 2011). Morah and Uduagwu (2017) studied the GC-MS of petroleum ether and ethanol extracts of the A. laxiflora leaves and identified twelve fatty acid derivatives, namely, ethyl linoleate, icosyl oleate, oleyl palmitoleate, methyl palmitate, ethyl palmitate, cyclopropanedodecanoic acid, 2-octyl-, methyl ester; methyl isostearate, 1-heptatriacotanol; ethanol, 2-(9,12-octadecadienyloxy)-, (Z,Z)-; 9-octadecene, 1,1′-[1,2-ethanediylbis(oxy)] bis-, (Z,Z)-; 9-desoxy-9x-chloroingol 3,7,8,12-tetraacetate and one lactone fatty acid ester tricyclo [20.8.0.0(7,16)]triacontane, 1(22),7(16)-diepoxy- (Table 2; Supplementary Figure S4). Moreover, Okokon et al. (2017b) also isolated thirteen saturated and unsaturated fatty acids and esters, namely, pentadecanoic acid, stearic acid, 2-hydroxyethyl oleate, henicosyl formate, methyl ricinoleate, methyl linoleate, methyl elaidolinolenate, 1,3-diacetyloxypropan-2-yl icosanoate, 1-tetradecanol, 1-hexadecanol, dimethyl undecanedioate, 1-heptacosanol, and Z,E-2,13-octadecadien-1-ol. Okokon et al. (2017a) isolated and characterized a multitude of fatty acids from A. laxiflora root ethyl acetate fraction, including ethyl oleate, methyl oleate, α-linoleic acid, propyl linoleate, methyl palmitate, ethyl palmitate, trimethylsilyl palmitate, stearic acid, ethyl stearate, ethyl tetracosanoate, pentadecanoic acid, ethyl ester, ethyl laurate, ethyl myristate, elaidic acid trimethylsilyl, and a lactone fatty acid ester 2H-pyran-2-one, tetrahydro-4-hydroxy-6-pentyl (Supplementary Figure S5).
Alkaloids
Various studies reported the presence of alkaloids in A. laxiflora using phytochemical screening, but only a few compounds have been isolated (Table 2; Supplementary Figure S6). An unusual prenylguanidinyl-epicatechin derivative alchornealaxine was separated from A. laxiflora leaves by Tapondjou et al. (2016). In another report, one pyrrolidine alkaloid, namely, 4-fluoro-2-nitroaniline, 5-[4-(pyrrolidin-1-yl) carbonylmethylpiperazin1-yl]-, was isolated from the extract of A. laxiflora leaves (Morah and Uduagwu, 2017). In two distinct studies by Okokon et al. (2017b, 2017a), two capsaicinoid alkaloids (capsaicin and dihydrocapsaicin) and one purine alkaloid called 1,3,7,9-tetramethyluric acid were isolated from the leaves and roots of A. laxiflora. Bafor et al. (2018) captured the porphine derivative pheophorbide A from the methanol A. laxiflora extract.
Miscellaneous compounds
Notable compounds isolated from A. laxiflora include megastigmane glycoside (byzantionoside B, leeaoside), carbohydrates (2-methylerythritol, 4-amino-4-deoxyarabinose, 3-deoxy-arabino-hept-2-ulosonic acid), amino acids (2-amino-4,5-dihydroxy-3,4-dimethylpentanoic acid), alkanes (octadecane, 3-ethyl-5-(2-ethylbutyl)-), and 2-methyl-3,5-dinitrobenzyl alcohol (Tapondjou et al., 2016; Morah and Uduagwu, 2017). Two distinct studies by Okokon et al. (2017b, 2017a) reported the GC and GC-MS analysis of ethyl acetate fraction from A. laxiflora leaves and roots. They discovered that the ethyl acetate fraction was dominated by multifarious bioactive compounds: 1-hexadecene, 1-octadecene, 1-tetradecene, D-galactitol,3,6-anhydro-1,2,4,5-tetra-O-methyl-; 10,11-dihydro-10-hydroxy-2,3-dimethoxydibenz(b,f)oxepin; hydroxy-4,4-dimethyldihydro-2(3H)-furanone; 2-coumaranone, 2-cyclopenten-1-one, 2-methyl-; (Z),(Z)-2,5-dimethyl-2,4-hexadienedioic acid; 1-octadecene, propanoic acid, 3-(trimethylsilyl)-, ethyl ester; 2-furancarboxylic acid, trimethylsilyl ester; cyclopropenoic acid,1-trimethylsilyl,-2-(2-methylpropen-1-yl), methyl ester; 2H-pyran-2-one, 5,6-dihydro-6-pentyl-, (R)-; 1,2,4-cyclopentanetrione, 3-butyl-; octadecane, 1-bromo-; 2-butenoic acid, 2-methoxy-3-methyl-, methyl ester; benzoic acid, 3-acetyloxy-, trimethylsilyl ester; cyclopropanecarboxylic acid, 2,2-dimethyl-3-cis-(2-methyl-3-buten-2-yl)-; 3-{[tert-butyl (dimethyl)silyl]oxy}butanal; benzeneacetic acid, alpha-[(trimethylsilyl)oxy]-; 2-hydroxy-3-methoxybenzaldehyde, trimethylsilyl ether and benzene, and (2-ethyl-4-methyl-1,3-pentadienyl)-, (E) (Table 2; Supplementary Figure S7).
This literature review reveals that A. laxiflora is rich in flavonoids, phenolic compounds, terpenoids, fatty acids, steroids, and alkaloids. To date, 132 compounds have been identified and structurally elucidated from the extracts of A. laxiflora, including 13 flavonoids, 19 phenolics, 22 terpenoids, 43 fatty acids, 6 alkaloids, and other secondary metabolites. Nonetheless, most of these compounds have been identified from the leaves, stems, and roots of this plant. Consequently, it is suggested to utilize inflorescence, flowers, and fruits to identify and isolate chemical constituents. Moreover, further research is required to establish the therapeutic applications of isolated compounds of this plant.
Pharmacological activity
Traditionally, the whole plant, leaves, roots, stem, and fruits of A. laxiflora are used to treat various complications in different regions of Africa (Figure 2). In particular, the Cameroonian traditional medicine system documented different applications of this plant as remedies for various health issues in Cameroonian traditional pharmacopeia (Sandjo et al., 2011). A wide range of pharmacological activities of the A. laxiflora extracts and its isolated phytochemicals have been reported using different in vitro and in vivo methods in the last 2 decades (Tables 3, 4 and Figures 4–6).
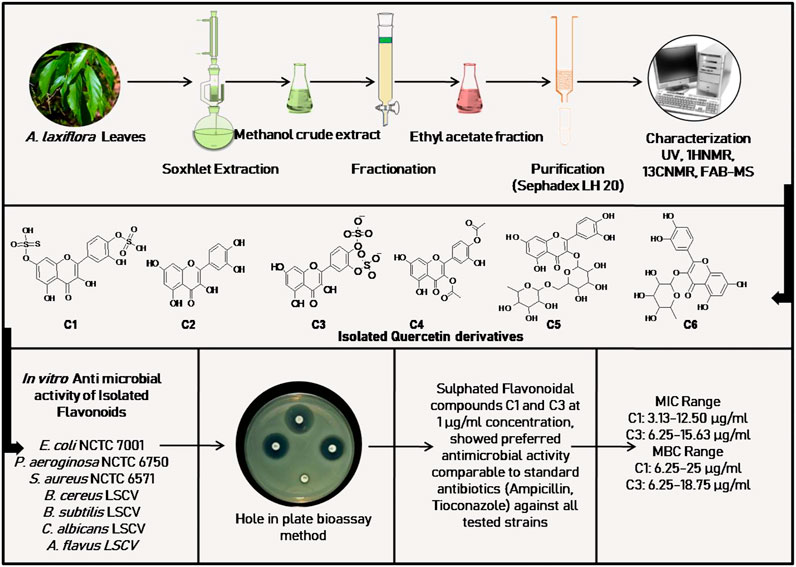
FIGURE 4. Anti-microbial activity of isolated flavonoids from A. laxiflora, derived from Ogundipe et al. (2001b).
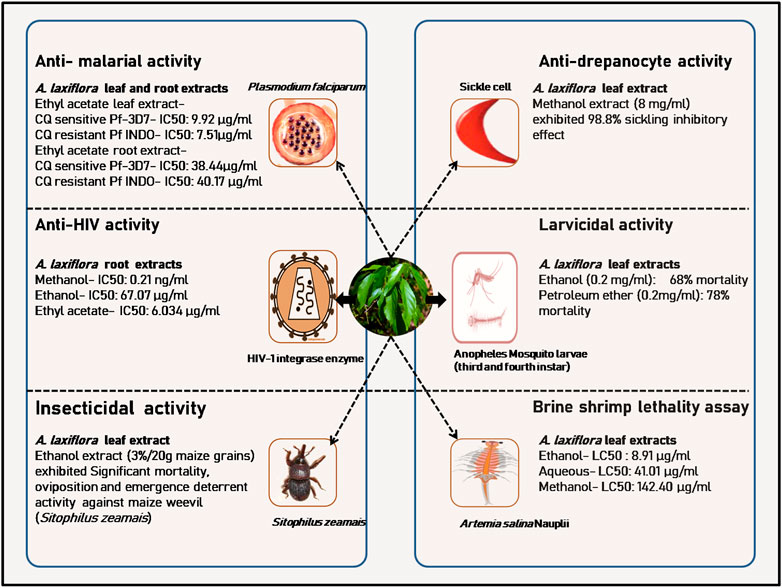
FIGURE 5. A few in vitro pharmacological and toxicological activities of A. laxiflora derived from Ogbole et al. (2016), Okokon et al. (2017a; 2017b), Morah and Uduagwu (2017), Osabiya et al. (2017), Bamimore and Elujoba (2018), Siwe-Noundou et al. (2019), and Ileke et al. (2020).
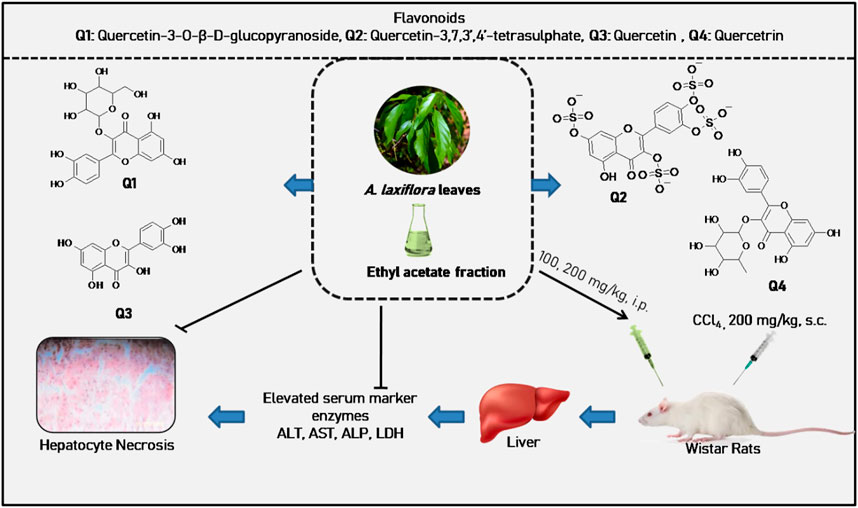
FIGURE 6. Hepatoprotective activity of A. laxiflora derived from Oloyede et al. (2011).
Antibacterial and antifungal activity
The most common ethnomedicinal use of A. laxiflora includes the treatment of various infectious diseases such as typhoid, diarrhea, urinary tract infections, and venereal diseases. Hence, the application of A. laxiflora as an anti-infective agent prompted (Ogundipe et al., 2001b) to carry out bioactivity-guided isolation of the active constituent of A. laxiflora. Ogundipe et al. (2001b) evaluated six flavonoids associated with ethyl acetate fraction of methanolic A. laxiflora leave extract, namely, quercetin-7,4′-disulphate, quercetin, quercetin-3′,4′-disulphate, quercetin-3,4′-diacetate, rutin, and quercitrin against Escherichia coli (E. coli) NCTC 7001, Pseudomonas aeruginosa (P. aeruginosa) NCTC 6750, Staphylococcus aureus (S. aureus) NCTC 6571, Bacillus cereus (B. cereus) LSCV, Bacillus subtilis (B. subtilis) LSCV, Candida albicans (C. albicans) LSCV, and Aspergillus flavus (A. flavus) LSCV using the broth dilution method for determining the MIC and MBC of isolates with gentamicin 2.5 μg/ml, ampicillin 2.5 μg/ml, and tioconazole 10 μg/ml as reference compounds. The sulfated quercetin derivates quercetin-7,4′-disulphate (MIC range 3.13–12.50 μg/ml) and quercetin-3′,4′-disulphate (MIC range 6.25–15.63 μg/ml) showed preferred antimicrobial activity against all microorganism species than quercetin (MIC range 62.5–120.2 μg/ml) and comparable activity with standard drug ampicillin and tioconazole. Unfortunately, rutin and quercitrin did not exhibit any activity in this study (Table 3; Figure 4).
Different extracts (hexane, ethyl acetate, and butanol) of A. laxiflora leaves were screened against S. aureus, E. coli NCIB 86, B. subtilis NCIB 3610, and P. aeruginosa NCIB 950 using the agar well-diffusion method with streptomycin as a control. The mean zone of inhibition (ZOI) ranged between 10.1 and 17.1 mm, equivalent to the solvents (hexane, ethyl acetate, and butanol) used in the study (Oloyede et al., 2010), thereby getting negative results. Similar findings were reported (Essiett and Ajibesin, 2010) for antimicrobial evaluation of ethanolic extract of leaves against six human pathogenic bacteria—S. aureus NCIB 8588, B. subtilis NCIB 3610, E. coli NCIB 86, Proteus vulgaris (P. vulgaris) NCIB 67, P. aeruginosa NCIB 950, and Klebsiella pneumoniae (K. pneumoniae) NCIB 418—and two clinical fungal isolates, C. albicans and A. flavus. The obtained results revealed that the extract exhibits moderate inhibition against P. aeruginosa NCIB 950, P. vulgaris NCIB 67, and A. flavus with ZOI of 15 ± 3.6 mm, 11 mm, and 9 mm, respectively. The highest MIC value of 250 µg/ml was observed against P. aeruginosa NCIB 950 and A. flavus. However, no activity was observed with other tested strains (four bacterial and one fungal) (Table 3).
Akinpelu et al. (2015) reported a broad-spectrum antimicrobial activity of hydroalcoholic extract from A. laxiflora leaves. The antibacterial and antifungal activity of the A. laxiflora extract was evaluated against a panel of bacterial (39) and fungal (17) isolates. The extract at a concentration of 25 mg/ml inhibited all the bacterial isolates, with the ZOI ranging between 12 and 24 mm and MIC ranging between 0.78 and 25 mg/ml. Similarly, at the concentration of 35 mg/ml, the A. laxiflora extract inhibited 15 isolates out of 17 fungal isolates, with ZOI ranging between 11 and 23 mm and MIC ranging between 8.75 and 35.00 mg/ml. The highest antibacterial, antifungal activity of A. laxiflora was observed against Shigella species (24 ± 0.50 mm; MIC 3.13 mg/ml) and Trichophyton tonsurans (T. tonsurans) (23 ± 0.50 mm; MIC 2.19 mg/ml), respectively.
Another investigation of the antibacterial activity of A. laxiflora was conducted by Mbaveng et al. (2015). The antibacterial activity of a novel flavonoid, 3,4,3′-tri-O-methylellagic acid, isolated from the stem bark of A. laxiflora was evaluated against a panel of 14 g negative multi-drug resistant (MDR) bacteria, including strains of E. coli (ATCC 8739, AG102, AG100 Atet), Enterobacter aerogenes (E. aerogenes) (ATCC 13048, CM64, EA27), K. pneumoniae (ATCC 11296, KP55), Providencia stuartii (P. stuartii) (ATCC29916, PS299645), Enterobacter cloacae (E. cloacae) (BM47, BM67), and P. aeruginosa (PA01, PA124) using chloramphenicol as a standard antibiotic. Compound 3,4,3′-tri-O-methylellagic acid exhibited weak antibacterial activity with MIC values ranging from 64 to 256 µg/ml on 4/14 (29%) and more than 256 µg/ml on 8/14 (57%), with no activity on the 2/14 (14%) tested bacteria. The lowest MIC value of 64 µg/ml was obtained against P. stuartii ATCC29916. Likewise, the methanolic extract from the leaves and stem bark of A. laxiflora was tested for their antibacterial activity against sensitive and resistant strains of bacteria, namely, P. aeruginosa (PA01, PA124), K. pneumoniae (ATCC11296, KP55, KP63), E. aerogenes (ATCC13048, CM64, EA 27, EA 289), E. coli (ATCC8739, ATCC10536, AG100ATet, AG102), and P. stuartii (ATCC29916, NEA 16) using rapid INT colorimetric assay. The result showed that except for E. coli AG100ATet and P. aeruginosa PA124, all other test strains (13/15, 86.7%) exerted sensitivity to methanolic leaves and stem bark extract of A. laxiflora with a MIC value range of 64–1,024 µg/ml. The A. laxiflora bark extract exhibited the highest antibacterial activity with a MIC value of 64 µg/ml against E. aerogenes EA 289 (Tchinda et al., 2017).
The different extracts (aqueous, ethyl acetate, and ethanol) of A. laxiflora leaves were tested against the clinical strains of Salmonella typhi (S. typhi) and Salmonella paratyphi (S. paratyphi) isolated from human stool. The extract showed dose-dependent inhibition in both the tested strains in different concentrations, likely 20, 40, and 60 mg/ml. However, ethyl acetate extract at the concentration of 60 mg/ml showed the highest antibacterial activity against S. typhi and S. paratyphi with ZOI of 24 and 19 mm, respectively (Osuntokun and Olajubu, 2015). Similar results were reported in another study by Wansi et al. (2017), in which the extract of methanol leaves exhibited preferred activity against S. typhi and Shigella flexneri (S. flexneri) with MIC values of 512 μg/ml and 1024 μg/ml, respectively.
Osabiya et al. (2017) demonstrated that aqueous and ethanol extracts from the leaves of A. laxiflora inhibited six bacterial strains, namely, B. subtilis, S. aureus, E. coli, Enterococcus faecalis (E. faecalis), K. pneumoniae, and S. typhi. The MIC ranged between 2.5 and 40 mg/ml. However, it was a noteworthy result that the ethanol extract exhibited more potent inhibitory activity at a concentration of 60 mg/ml against S. aureus (19.33 ± 0.58 mm) and K. pneumoniae (18.33 ± 0.58 mm) compared with the antibiotic chloramphenicol (11.67 ± 0.57 mm and 10.01 ± 0.00 mm). Furthermore, in vitro antibacterial activity of essential oils from leaves of A. laxiflora was carried out against B. cereus ATCC 10872, B. subtilis ATCC 6633, S. aureus ATCC 25923, P. aeruginosa ATCC 9027, and E. coli ATCC 25922. The mean ZOI ranged between 7 and 9 mm in contrast to the standard antibiotic ampicillin, with ZOI ranging between 6 and 8 mm (Otuechere et al., 2019).
On account of the traditional use of A. laxiflora leave decoction and infusion for the treatment of digestive and gastric disorders, Ngnameko et al. (2019) investigated the anti-Helicobacter pylori (H. pylori) activity of A. laxiflora leave extract. The extract was active against H. pylori at a MIC of 20 mg/ml. Lately, in vitro antibacterial activity of hexane, chloroform, ethyl acetate, methanol, ethanol, and aqueous extracts of the leaves, roots, and stem bark of A. laxiflora on the skin, gastrointestinal, respiratory, and urinary pathogens was also conducted. The extracts were tested against four Gram-positive bacteria, namely, B. cereus ATCC 11778, E. faecalis ATCC 29212, S. aureus ATCC 25923, and Staphylococcus saprophyticus (S. saprophyticus) ATCC 15305, and four Gram-negative bacterial strains, namely, E. coli ATCC 25922, K. pneumoniae ATCC 13883, Moraxella catarrhalis (M. catarrhalis) ATCC 23246, and Proteus mirabilis (P. mirabilis) ATCC 43071 using ciprofloxacin as a standard antibacterial agent. All the extracts were effective against most of the tested Gram-positive strains, with MIC ranging between 50 and 63 μg/ml. In addition, bioactivity-guided fractionation of methanolic extract of the A. laxiflora stem results in the isolation of ellagic acid, 3-O-methylellagic acid, 3-O-β-D-glucopyranosyl-β-sitosterol, 3-O-acetyl-oleanolic acid, and 3-O-acetyl-ursolic acid. All the compounds displayed antibacterial activity against tested strains with MIC values as low as 4 μg/ml (Siwe-Noundou et al., 2019) (Table 3).
Based on the literature survey, certain gaps were identified in the reported studies. For example, Osabiya et al. (2017) did not mention the strain collection numbers of investigated bacterial strains, which makes it difficult to establish a comparison with other studies. Similarly, limitations were observed in the studies reported by Ngnameko et al. (2019), Osuntokun and Olajubu (2015), and Wansi et al. (2017). For the purpose of quality control, the European Committee on Antimicrobial Susceptibility Testing (EUCAST) has recommended strain collection numbers from the distributors such as ATCC (American Type Culture Collection, United States), NCTC (National Collection of Type Cultures, United Kingdom), CIP (Collection de Institut Pasteur, France), CECT (Coleccion Espanola de Cultivos Tipo, Spain), CCUG (The Culture Collection University of Gothenburg, Sweden), and DSM (Deutsche Stammsammlung fur Mikroorganismen und Zellkulturen, Germany) (Matuschek et al., 2014). Furthermore, the assessment of anti-microbial activity using only the disc diffusion method is a preliminary approach because it did not ascertain the exact concentration-causing antimicrobial effect. Oloyede et al. (2010) reported the ZOI of different extracts determined by the disc diffusion method only. Unfortunately, they did not use agar or broth dilution tests to determine the MIC values. Moreover, the use of the agar diffusion method to determine the antimicrobial activity of plant extracts is considered inadequate owing to the lack of diffusion of non-polar molecules into the aqueous agar matrix, insensitivity, and non-reproducibility of the results in different laboratories. Therefore, serial microplate dilution methods using INT or rezurasin as indicators of growth are the preferred methods to determine realistic and reproducible MIC values (Eloff, 2019). Only a few studies reported the outcomes of rapid INT colorimetric assay. Plant extracts displaying MIC value ≤100 μg/ml are considered to possess noteworthy antimicrobial activity (Bueno, 2012). Nonetheless, multiple studies included in this review have reported MIC values higher than the accepted limit of ≤100 μg/ml. It is often not determined whether the antimicrobial activity is caused by general toxicity to all cells or a selective activity against the microorganisms.
Anti-amoebic and anti-plasmodial activity
Moundipa et al. (2005) investigated the in vitro amoebicidal activity of methanolic leave extract of A. laxiflora against clinical isolates of Entamoeba histolytica (E. histolytica). The extract, at a concentration of 100 μg/ml, showed 60.43% and 52.17% mortality on day 2 and day 4, respectively, indicating anti-amoebic activity.
Okokon et al. (2017b) investigated the in vitro antiplasmodial activity of A. laxiflora leaves, crude ethanol, petroleum ether, chloroform, ethyl acetate, and butanol extracts against two Plasmodium falciparum (P. falciparum) strains: CQ sensitive Pf-3D7 and CQ resistant Pf INDO SYBR green assay method. The crude ethanol, petroleum ether, chloroform, ethyl acetate, butanol, and aqueous leave extract of A. laxiflora were found to be active in vitro with CQ-sensitive IC50 values of 31.57 ± 0.94, 27.85 ± 0.36, 26.06 ± 0.19, 9.92 ± 0.28, >100, >100 μg/ml, respectively, and had CQ-resistant IC50 values of 16.38 ± 0.94, 23.47 ± 0.15, 14.47 ± 0.35, 7.51 ± 0.24, 52.63 ± 0.22, and >100 μg/ml, respectively. However, the ethyl acetate fraction exhibited the most promising activity against both strains of P. falciparum. Further, the fractionation of ethyl acetate extract led to the isolation of 34 phytoconstituents, including polyunsaturated fatty acids (PUFA), phenolics, and flavonoids. Likewise, prophylactic, suppressive, and curative effects of ethanol A. laxiflora leave extract (200–600 mg/kg, p. o.) were tested in vivo using Plasmodium berghei (P. berghei) infected mice. The extract showed promising antimalarial activity (Table 4; Figure 5). Similar results were reported by Okokon et al. (2017a) with the A. laxiflora root extract. The root extract and fractions also exerted moderate activity against CQ-sensitive (Pf 3D7) and CQ-resistant (Pf INDO) strains of P. falciparum, with ethyl acetate fraction exerting the highest activity with an IC50 value of 38.44 ± 0.89 and 40.17 ± 0.78 μg/ml in Pf 3D7 and Pf INDO strains, respectively. Recently, an in vivo antiplasmodial activity of methanol and chloroform leave extract of A. laxiflora was reported (Table 4; Figure 5). Briefly, the extract was administered orally (200–600 mg/kg) to P. berghei-infected mice. After 5 days of the treatment study, methanol extract exhibited significantly higher prophylactic, suppressive, and curative activity than the chloroform extract. These studies confirmed the ethnopharmacological use of A. laxiflora as a promising indigenous antimalarial drug (Oluyemi and Blessing, 2019) (Table 4).
Larvicidal activity
Morah and Uduagwu (2017) investigated the larvicidal activity of ethanol and petroleum ether extracts from the leaves of A. laxiflora against the third and fourth instar Anopheles mosquito larvae. Larva mortality was observed for both petroleum ether and ethanol extract at all concentrations assayed (0.08, 0.1, 0.15, 0.2 mg/ml), with the lowest activity at 0.08 mg/ml and the highest mortality rate observed at 0.2 mg/ml. Moreover, petroleum ether extract exhibited the highest 78% mortality compared to 68%, by ethanol extract at a similar dose of 0.2 mg/ml. In contrast, the larvicidal activity was attributed to the presence of methyl palmitate, icosyl oleate, and diisooctyl phthalate (Table 4; Figure 5).
Anti-HIV potential
Various extracts of A. laxiflora leaves, stem, and roots were evaluated for their Anti-HIV activity using HIV-1 integrase strand transfer assay. HIV-1 integrase inhibitory activity dwelt in most of the root extracts of A. laxiflora. The methanolic extract of A. laxiflora root exhibited noteworthy HIV-1 integrase inhibitory activity at an IC50 value of 0.21 ng/ml compared to chicoric acid taken as a reference (IC50 = 6.82 μM) without any significant cytotoxicity against HeLa cells. The ethanolic root extract and ethyl acetate root extract also exhibited marked HIV-1 integrase inhibitory activity with IC50 values of 67.07 and 6.034 μg/ml, respectively. Moreover, the inhibitory activity of five compounds (ellagic acid, 3-O-methylellagic acid, 3-O-β-D-glucopyranosyl-β-sitosterol, 3-O-acetyl-oleanolic acid, and 3-O-acetyl-ursolic acid) isolated from the methanolic stem extract of A. laxiflora was also investigated on HIV-1 integrase, although all the isolated compounds were non-cytotoxic. However, they did not exhibit significant anti-HIV-1 integrase activity. Only ellagic acid laid out the best HIV-1 integrase inhibitory activity with an IC50 value of 90.23 μM. The IC50 values of other isolated compounds (3-O-methylellagic acid, 3-O-β-D-glucopyranosyl-β-sitosterol, 3-O-acetyl-oleanolic acid, and 3-O-acetyl-ursolic acid) were either >100 μM or could not be determined (Siwe-Noundou et al., 2019) (Table 4; Figure 5).
Antidiabetic activity
The use of A. laxiflora leave decoction for treating diabetes is common among the Badagry people of Nigeria. This practice was validated through in vitro and in vivo studies. Ogbole et al. (2016) showed that methanolic extract obtained from A. laxiflora leaves inhibits α-amylase moderately with an IC50 value of 295.60 μg/ml. The anti-diabetic properties of A. laxiflora have recently been studied using an in vivo alloxan-induced diabetic rat model. When methanolic extract of A. laxiflora leaves was administered orally at a dose of 500 mg/kg, it showed a significant (p < 0.05) anti-hyperglycemic effect by lowering blood glycemic levels in diabetic rats (Nimenibo-uadia, 2018) (Table 4).
Analgesic and anti-inflammatory activity
In order to substantiate the folklore medicinal use of A. laxiflora roots infusion or decoction as treatment of malaria and associated pain-related symptoms, Okokon et al. (2017a) investigated the analgesic activity of the crude ethanol extract and other solvent fractions of A. laxiflora using mice against the standard drug acetylsalicylic acid (ASA; 100 mg/kg) by acetic acid-induced writhing, hot plate, and formalin-induced hind paw licking methods. The administration of A. laxiflora root extract ((75, 150, and 225 mg/kg, p.o.) and extract fractions (150 mg/kg, p.o.) demonstrated a significant (p < 0.05–0.001) and dose-dependent analgesic activity in all used pain models. The ethyl acetate fraction exerted the highest analgesic activity compared with the standard drug ASA. The aqueous and methanol extracts from A. laxiflora leaves were appraised for analgesic effect in mice. The hot plate and tail immersion tests were used to evaluate the central analgesic effects, whereas the acetic acid-induced abdominal writhing assay was used to evaluate peripheral analgesic activity. The extract increased the mean reaction time to pain significantly in the hot plate and tail immersion tests. Additionally, the number of abdominal writhes also decreased substantially, endorsing the folklore use of this plant (Nwonu et al., 2018a).
The anti-inflammatory activity of acetone extract from A. laxiflora leaves was investigated in vitro. In the study, A. laxiflora extracts exerted dose-dependent inhibition of NO production by LPS-activated malignant macrophage cell line RAW264.7 at doses 6.25, 12.5, 25, and 50 μg/ml. At the concentration of 25 μg/ml, the extract exhibited the highest percent inhibition (96.53%). Simultaneously, the A. laxiflora extract significantly inhibited 15-LOX activity with an IC50 value of 46.03 μg/ml compared to quercetin used as a reference drug (35.85 μg/ml) (Dzoyem and Eloff, 2015). Furthermore, Ngoungoure et al. (2019) reported that methylene chloride/methanol (1:1; v/v) extract obtained from leaves of A. laxiflora demonstrated similar results. The A. laxiflora extract exerted 68.10% NO inhibition (IC50 = 66.57 μg/ml) and 54.58% 15-LOX inhibitory activity (IC50 = 90.42 μg/ml) (Table 4).
Anti-diarrheal activity
To substantiate the ethnomedicinal use of A. laxiflora leaves in Cameroon to treat some gastrointestinal disorders, Wansi et al. (2017) conducted an in vivo study to appraise the antidiarrheal activity of methanolic and aqueous extracts of A. laxiflora (125, 250, and 500 mg/kg) using S. flexneri-induced infectious, castor oil-induced secretory, and magnesium sulphate-induced osmotic diarrhea in rats. The methanolic extract showed promising antidiarrheal activity in all animal models. The A. laxiflora methanolic extract significantly prolonged the latency period at all doses in the model of osmotic diarrhea, with the 250 mg/kg dose giving the highest 71.6% prolongation in latency time (Table 4).
Hepatoprotective activity
In the Bamun folk medicine (Cameroon), A. laxiflora is reported to treat hepatitis and other liver-related disorders. As toxic hepatitis is often associated with the oxidative destruction of lipids and proteins, Njayou et al. (2008) carried out ex vivo rat liver microsomal lipid peroxidation (enzymatic and non-enzymatic) and protein oxidation inhibitory potential of methanol-methylene chloride extract of A. laxiflora leaves. On the whole, the A. laxiflora extract inhibited the biochemical process in a dose-dependent manner (10, 100, and 200 μg/ml) with the significant inhibition of microsomal lipid peroxidation (95.90 ± 0.57% and 79.17 ± 1.57%; enzymatic and non-enzymatic, respectively) and protein oxidation (95.60 ± 0.59%) at 200 μg/ml concentration.
The ethyl acetate extract of A. laxiflora was studied for its possible hepatoprotective effect against CCl4-induced hepatotoxicity in rats to rationalize some folklore use. The ethyl acetate extract of A. laxiflora at 100 mg/kg body weight significantly counteracted the CCl4-induced liver damage by lowering the elevated marker enzymes, namely, ALT, AST, ALP, and LDH levels in the blood to 18.872, 7.054, 22.864, and 180.321, respectively, compared to CCl4 elevated levels of 35.712, 12.513, 27.509, and 480.312 for ALT, AST, ALP, and LDH, respectively. Further, histopathological analysis of the liver showed that the ethyl acetate extract may protect the liver from centrilobular necrosis, vacuolization, and macrovesicular fatty change set up by CCl4. The hepatoprotective activity was ascribed to four isolated flavonoids, namely, quercetin, quercetin-3,7.3′,4′-tetrasulphate, quercetin-3-O-β-D-glucopyranoside, and quercitrin (Oloyede et al., 2011) (Table 4; Figure 6). In a separate study, A. laxiflora was tested for its ability to alleviate sodium arsenate-induced liver damage in Wistar rats. The hexane extract of the A. laxiflora leaves could significantly reverse the liver damage caused by sodium arsenate (Esosa et al., 2013). Similarly, the methanolic extract of A. laxiflora leaves significantly modulated the levels of liver biochemical parameters GGT, GST, ALT, and AST (Uhunmwangho et al., 2016). The hepatoprotective activity of A. laxiflora roots has recently been investigated using sodium arsenate-induced hepatotoxicity as a model. The hexane root extract could significantly lower the liver enzymes (ALT, AST, and ALP), liver metabolizing enzymes (NAD, GST, and Cytochrome b5), and other biochemical parameters (total protein, albumin level, and globulin) (Uhunmwangho et al., 2018). These findings backed up the folklore’s claim that this botanical drug can help with liver problems. (Table 4).
Anti-anemic activity
Oladiji et al. (2014) investigated the anti-anemic potential of aqueous leave extract of A. laxiflora at doses 100, 200, and 300 mg/kg, p.o. on hematological indices (PCV, Hb, RBC, MCV, MCH, and MCHC) of iron-deficient rats. The extract could significantly increase the hematological indices at all doses, compared with the reference drug ferrous sulfate and iron-sufficient rats, attesting to its folklore medicinal use in the treatment of anemia. Similar results were reported by Bada et al. (2017) with an ethanolic extract of A. laxiflora leaves. Another study was conducted on the anti-sickle cell anemic activity of A. laxiflora. The Anti-sickling activity of methanolic extract from A. laxiflora leaves was reported by Bamimore and Elujoba (2018). The extract had 98.8% sickling inhibitory action when given at a level of 8 mg/ml (Table 4; Figure 5).
Tocolytic and fertility-promoting activity
Traditional African medicine uses the leaf of A. laxiflora to treat various reproductive system diseases, including preterm labor, miscarriage, menstrual disorders, postpartum discomfort, fibroids, and infertility. To appraise the ethnomedicinal use of A. laxiflora leaves to prevent preterm labor or miscarriage, Bafor et al. (2015) investigated the effect of methanolic extract of A. laxiflora leaves on female reproductive structures. Briefly, non-pregnant female mice were orally administered methanolic leave extract (100 and 1,000 mg/kg) for 6 days, using progesterone (10 mg/kg s.c.) as a positive control. The results revealed that the A. laxiflora extract at a lower dose (100 mg/kg) exerted progesterone-like activity on the ovaries, uterus, and cervical glands asserting its folklore use in maintaining pregnancy. In another investigation, ex vivo uterine contraction modulatory activity of methanolic extract of A. laxiflora was conducted using uterine tissue preparation isolated from female albino mice. The extract significantly inhibited spontaneous, oxytocin, and potassium chloride-induced uterine contractions, possibly via calcium and potassium ions channel interaction. Furthermore, the authors also isolated three important bio-constituents, namely, 3-deoxy-arabino-hept-2-ulosonic acid, 17-hydroxyingenol, and pheophorbide A, possibly contributing to the methanolic A. laxiflora extract activity (Bafor et al., 2018).
Uhunmwangho et al. (2016) studied the effect of the methanolic A. laxiflora leave extract on CCl4-induced reproductive toxicity. The oral administration of the extract at a graded dose (0.1, 0.5, 1.0, 10.0, and 50 mg/kg body weight) in male Wistar rats for 7 days, significantly reversed the toxic effects of CCl4 and aided in male fertility by significantly increasing the percentage motility of sperm and inhibiting sperm morphological aberrations compared with positive control (normal saline) (Table 4).
Anticonvulsant and sedative activity
The leaves of A. laxiflora are traditionally used in the form of decoction or maceration to treat epilepsy and sleeplessness. Hence, the in vivo anti-convulsant and sedative activity of A. laxiflora was evaluated by Bum et al. (2009). The aqueous extract of the leaves of A. laxiflora was investigated for anticonvulsant and sedative activity in male Swiss mice using MES, PTZ, NMDA, INH, PIC, and STR-induced convulsions or turning behavior and diazepam sleep-induced animal models. The aqueous extract at a dose of 60 mg/kg protected 100% of mice against NMDA-induced turning behavior, and at a dose of 120 mg/kg, it protected 75% of mice against STR-induced seizures. The A. laxiflora extract failed to provide any significant protection against MES, PTZ, INH, and PIC-induced seizures. Moreover, in the diazepam-induced sleep test, A. laxiflora was insignificant in modifying the sleep duration of the control group, indicating the non-sedative activity of A. laxiflora. However, a recent study by Nwonu et al. (2018b) demonstrated the anxiolytic and sedative effects of aqueous and methanol extracts of A. laxiflora leaves using a staircase climbing test in mice. The results showed that both the methanol and the aqueous extracts of A. laxiflora leaves had significant sedative activity at a high dose (800 mg/kg) by significantly decreasing staircase climbing (Table 4).
Anti-Alzheimer activity
The cholinergic deficit is implicated in the pathogenesis of neurodegenerative disorders such as Alzheimer’s disease and associated progressive memory loss and cognitive function impairments. The cholinergic deficit is the result of a reduction in central nervous system ACh activity due to the AChE-related breakdown of ACh. The extracts from different parts of A. laxiflora have been screened for AChE and BuChE inhibitory activity. The first report is that of Dzoyem and Eloff (2015), reporting the AChE inhibition activity of the acetone extract of A. laxiflora leaves using the modified Ellman method. The extract exhibited significant but weak inhibitory activity with an IC50 value of 364.12 ± 2.39 μg/ml compared to eserine with an IC50 value of 4.94 ± 0.015 μg/ml. Similarly, the methylene chloride: methanol (1:1; v/v) extract at a single concentration of 200 μg/ml exhibited 36.02 ± 0.18% AChE inhibitory activity with >200 μg/ml, IC50 value compared to galantamine (100 μg/ml; IC50 value 24.65 ± 2.12 μg/ml); a standard drug (Ndam Ngoungoure et al., 2019). Elufioye (2017) investigated the effects of various extracts (hexane, ethyl acetate, and aqueous) obtained from A. laxiflora leaves, stem bark, and root bark in inhibiting both AChE and BuChE. The results reported that the A. laxiflora stem bark and root bark extracts showed selective AChE inhibitory activity with percent inhibition ranging from 10.69% to 34.20% (Table 4).
Antipsychotic activity
In vivo antipsychotic effects of aqueous and methanol extracts of A. laxiflora leaves were evaluated by Nwonu et al. (2018c). According to their findings, oral administration of the aqueous and methanol extracts in mice at graded doses (100, 200, 400, 800, and 1,600 mg/kg, p.o.) significantly reduced apomorphine-induced climbing and stereotypic behavior in mice at all tested doses, compared with chlorpromazine, a psycholeptic agent (Table 4).
Anti-Parkinson’s disease activity
Ngoungoure et al. (2019) assessed whether A. laxiflora methylene chloride: methanol (1:1; v/v) leave extract with antioxidant and anti-inflammatory activities could serve as a protective agent against aminochrome-induced toxicity in human astrocytoma cells (U373MG and U373MGsiGT6 cell lines). The results indicated that A. laxiflora extracts at doses 0.1 and 1.0 μg/ml significantly altered the aminochrome-induced (75 μM) cell death and mitochondrial membrane potential reduction in both cell lines, implicating the potential usefulness of A. laxiflora in Parkinson’s disease. However, further studies are warranted in isolating the active constituents and detailed elucidation of the mechanism of the action for safe and effective utility in Parkinson’s disease (Table 4).
Anxiolytic activity
The anti-anxiety efficacy of aqueous and methanol extracts of A. laxiflora in mice was tested using the elevated plus-maze and the staircase behavioral paradigms to indicate the ethnomedicinal usage of A. laxiflora as an anxiolytic drug (Nwonu et al., 2018b). A. laxiflora exerted a significant anxiolytic effect on the elevated plus maze and staircase animal model against the standard drug diazepam (0.1 mg/kg, p.o.). Albino mice were divided into seven groups of six animals each. The control group (I) received 10% Tween-80 (10 ml/kg, p.o.), whereas the test groups (II–VI) received A. laxiflora aqueous or methanol leave extract in graded doses (100, 200, 400, 800, 1,600 mg/kg, p.o.) and standard drug group (VII) received standard drug diazepam (1 mg/kg i.p.) 30 or 60 min before the experiments. In the elevated plus maze model, the A. laxiflora methanol extract significantly increased the percent entry and percent time spent in open arms at lower and higher doses (100, 200, 800, and 1,600 mg/kg). In contrast, almost all doses significantly decreased the index of open-arm avoidance, attesting to anxiolytic activity. Similar results were observed for the aqueous extract of A. laxiflora leaves at doses 400 and 1,600 mg/kg, p.o., validating its anti-anxiety effect. In the staircase paradigm also, the methanol and the aqueous extracts of A. laxiflora leaves exhibited anti-anxiety activity by decreasing staircase rearing (aqueous extract: all doses; methanol extract: 400, 800, and 1,600 mg/kg, p.o.) and increasing staircase step-climbing (aqueous extract: 200 and 800 mg/kg p.o.; methanol extract: 100 and 200 mg/kg, p.o.) behaviors significantly (Table 4).
Antioxidant activity
In the Ugba region of Nigeria, the leaves of A. laxiflora are traditionally used to wrap food items for preservation. Farombi et al. (2003) were the first to report the antioxidant activity of hexane and methanol extracts from A. laxiflora leaves and roots using the ferric thiocyanate method, horseradish peroxidase catalyzed oxidation of ABTS, β-carotene linoleate model system, and rat liver microsomal lipid peroxidation assay. The antioxidant activity was observed in the following order: hexane root extract (76.4%) > methanol root extract (63%) > methanol leave extract (40%) > hexane leave extract (38%) at 0.05% concentration. The hexane root extract’s antioxidant activity was compared with that of BHA (80%), a standard antioxidant. Another report indicated the antioxidant activity of hydroethanolic extract and solvent fractions of A. laxiflora leaves by the DPPH spectrophotometric assay method. All the test samples showed less scavenging activity (EC50 12.97, 24.34, and 106.74 μg/ml for ethyl acetate, n-butanol, and crude ethanol extract, respectively) relative to the reference standard ascorbic acid (EC50 4.78 μg/ml). Moreover, bioassay-guided fractionation of n-butanol fraction led to the isolation of two flavonoids, namely, taxifolin glycoside and quercitrin, suggesting their involvement in observed antioxidant activity (Adeloye et al., 2005).
According to Oloyede et al. (2010), the H2O2 scavenging activity of the butanol extract of A. laxiflora through the FTC method was compared favorably with standard reference α-tocopherol at 500 μg/ml concentration. The scavenging activity was observed for the various extracts (hexane, ethyl acetate, butanol, and aqueous) of leaves at various concentrations (50, 100, 250, and 500 μg/ml) assayed in a concentration dependant manner. Similarly, the acetone leave extract showed significant (p < 0.05) antioxidant activity in DPPH, ABTS, and FRAP assays with IC50 values of 17.19 ± 1.02, 18.53 ± 1.42, and 438.42 ± 15.55 μg/ml, respectively. Trolox was used as a standard antioxidant with IC50 values of 3.14 ± 0.10 and 6.05 ± 0.24 μg/ml in DPPH and ABTS assay, respectively (Dzoyem and Eloff, 2015). The in vivo antioxidant assay of methanolic extract was investigated by determining the effects on serum CAT, SOD, and GSH enzymes in experimental animals. The extract at different concentrations (0.5, 1.0, and10.50 mg/kg body weight, p.o.) significantly (p < 0.05) raised the GSH, SOD, and CAT activity (Uhunmwangho et al., 2017). Lately, Morah and Uduagwu (2017) assessed the radical scavenging activity of petroleum ether and ethanol extract of A. laxiflora using a DPPH spectrophotometric assay. Both extracts at all concentrations (40, 80, 100, 150, and 200 μg/ml) exhibited a dose-dependent anti-DPPH activity. The petroleum ether and ethanol extract at 200 μg/ml concentration exhibited greater percent DPPH radical scavenging ability (50.50% and 42.95%, respectively) than ascorbic acid at the same concentration (35.22%). The antioxidant activity of extracts was attributed to the isolated compounds, namely, 3-acetoxy-7,8-epoxylanostan-11-one, rhodopin, ethyl iso-allocholate, hexadecanoic acid, 9-octadecenyl hexanoate, eicosyl oleate, and astaxanthin. The results laid the credence for the ethnobotanical food preservative and natural oxidant use of A. laxiflora (Table 4).
Anti-cancer activity
Although A. laxiflora plant parts are traditionally used as an alternative medicine for cancer treatment, there is a paucity of experimental and clinical data on the anticancer activity of A. laxiflora (Dzoyem and Eloff, 2015). In the brine shrimp bioassay against Artemia salina, the aqueous, ethanol, and methanol extract of A. laxiflora was found toxic with LC50 values of 41.01, 8.91, and 142.40 μg/ml, respectively, indicating potential anti-cancer properties (Ogbole et al., 2016; Osabiya et al., 2017) (Figure 5). The methanolic extract of A. laxiflora root, stem, and leaves had significant anticancer activity against drug-sensitive leukemia CCRF-CEM cell line with IC50 values of >80, 49.21 ± 11.16, and 43.67 ± 4.06 μg/ml, respectively (Kuete et al., 2016). In two separate studies, Okokon et al. (2017a, 2017b) investigated the cytotoxicity activity of ethanol extract and solvent fractions (petroleum ether, dichloromethane, ethyl acetate, butanol, and aqueous) of A. laxiflora leaves and root against HeLa and HEK cell lines using MTT assay. The A. laxiflora root crude ethanolic extracts and fractions were non-toxic in the HeLa cell line with an IC50 value greater than 100 μg/ml, whereas the A. laxiflora root extracts and fractions were cytotoxic to the tested HeLa and HEK cell lines with potency order of ethyl acetate > chloroform > petroleum ether > crude extract. The ethyl acetate fraction was highly toxic to both cell lines with TC50 values of 8.83 and 1.41 μg/ml, respectively, suggesting ethyl acetate fraction could be a potential source of anticancer agents. In contrast, Siwe-Noundou et al. (2019) reported that various extracts (hexane, chloroform, ethyl acetate, methanol, ethanol, and aqueous, 25 μg/ml) of A. laxiflora root, stem, and leaves and five isolated compounds from methanolic A. laxiflora stem bark, namely, ellagic acid, 3-O-methylellagic acid, 3-O-β-D-glucopyranosyl-β-sitosterol, 3-O-acetyl-oleanolic acid, and 3-O-acetyl-ursolic acid (20 μM), were non-toxic against HeLa cell lines (cell viability >100%). Sandjo et al. (2011) tested the cytotoxicity of six isolated compounds, namely, (10Z)-tetradec-10-enoic acid-(2S)-2-carboxy-2-hydroxyethyl ester; (2R)-2-hydroxy-N-[(2S,3S,4R,15Z)-1,3,4-trihydroxy-15-triaconten-2-yl]octacosamide; 3-acetyloleanolic acid; 3-acetoxyursolic acid; 3-O-methylellagic acid; and 3-O-methylellagic acid-3′-O-α-rhamnopyranoside, against the HL60 cell line. Compounds 3-acetyloleanolic acid and 3-acetoxyursolic acid exhibited potent cytotoxic effects with IC50 values of 6.6 and 6.8 μM, respectively (Table 4).
Though multiple studies have focused on the pharmacological role of A. laxiflora, some parts of A. laxiflora, such as stem branchlets, fruits, and seeds, have not yet been studied. Indeed, fruits of this plant are used in traditional medicine for curing infertility and fibroid treatment in women. Hence, it is highly recommended to conduct further research in this domain. Moreover, most of the research focuses on in vitro pharmacology of A. laxiflora. Thus, researchers are strongly recommended to conduct in vivo pharmacological and clinical studies to understand the molecular mechanism of action of this botanical drug.
Toxicity studies
Only a few researchers have evaluated the toxicity of A. laxiflora. Farombi et al. (2003) investigated the acute toxicity of the hexane and methanol extract of A. laxiflora roots and leaves by administering it in Wistar rats by oral route (100–5,000 mg/kg). After 14 days of monitoring, the extract did not affect mortality. In rats given the drug orally, the LD50 was greater than 5,000 mg/kg.
The protective effects of hexane leaf extract of A. laxiflora against sodium arsenate-induced (2 mg/kg, p.o.) liver toxicity in Wistar rats was investigated by Esosa et al. (2013). The rats were treated with sodium arsenate for 2 days and suffered elevation in serum and liver biochemical indices (ALT, AST, ALP, GGP, and TB). Pretreatment with the extract of A. laxiflora hexane leaves at 10 mg/kg as prophylaxis significantly reversed these changes. Though, post-treatment of animals with the extract after a sodium arsenate treatment did not completely normalize the biochemical indices, suggesting that A. laxiflora extract may protect against sodium arsenate-induced hepatic toxicity.
The toxicity of the methanol extract of A. laxiflora leaves was evaluated by brine shrimp lethality bioassay. The methanolic extract displayed significant lethality with an LC50 value of 142.40 μg/ml (Ogbole et al., 2016). In the same line, Osabiya et al. (2017) reported the high toxicity potential of ethanol and aqueous leaf extracts of A. laxiflora by brine shrimp lethality bioassay. The aqueous and ethanol extracts showed strong toxicity with LC50 values of 41.01 and 8.91 μg/ml, respectively (Figure 5).
In two separate studies, Okokon et al. (2017a, 2017b) examined the possible development of toxicity caused by ethanol extracts of A. laxiflora leaves and roots by administering various doses of extracts intraperitoneally in albino mice. The LD50 values were 2,236 and 748.33 mg/kg for leaves and root extract, respectively, indicating that the ethanolic extract might be toxic at higher doses when administered intraperitoneally.
The acute toxicity of the A. laxiflora aqueous and methanolic leave extract was assessed using albino mice. In this study, the aqueous extract was well tolerated by the animals up to 1,600 mg/kg (oral, intraperitoneal), whereas the methanol extract was safe up to 400 mg/kg and 1,600 mg/kg, intraperitoneally and orally, respectively (Nwonu et al., 2018b; 2018c; 2018a).
In a 21-day study, the effect of ethanolic extract from A. laxiflora was assayed on hematological indices and organ body weight of Wistar rats. The extract had an anemia-ameliorative effect by increasing the RBC, WBC, platelets, PCV, and Hb levels. However, there was an increase in the heart and lung weights at higher doses (200 and 300 mg/kg, p.o.), showing that the plant should be used cautiously when used orally at higher doses for the treatment of anemia (Bada et al., 2017).
Essential oils from A. laxiflora leaves obtained by hydro-distillation were also assessed for toxic effects at doses 100, 200, and 400 mg/kg on albino rats. Oral administration of essential oils at higher doses (200 and 400 mg/kg) elevated serum ALT activity, depletion of serum bilirubin, and liver hypertrophy, suggesting possible derangement of hepatic functions at higher doses (Otuechere et al., 2019).
A few reports have highlighted the toxicological aspects of A. laxiflora. Although in vitro and in vivo models have been utilized, only acute effects have been studied. Sub-acute, chronic, mutagenic, and teratogenic effects need to be assessed to support the safe use of this plant. Moreover, the toxicity analysis of chemical constituents is recommended to be carried out rather than its crude extract.
Clinical studies
Chewing A. laxiflora sticks are common oral cleaning practices in Nigeria (Odugbemi and Akinsulire, 2008), considered a good source of fluoride, an essential element for preventing dental caries. To rationalize A. laxiflora use as chewing sticks as a viable alternative in providing the required fluoride in poor communities, Emeke et al. (2019) determined and compared the salivary fluoride retention after the A. laxiflora stick used with a non-herbal fluorinated product by using a double blind cross over experimental study with 20 participants. Salivary fluoride concentration was determined after 0, 10, 30, 45, and 60 min after chewing stick use. In the results, the baseline salivary fluoride concentration was 25.95 ± 4.58 ppm, whereas after A. laxiflora use, the salivary fluoride concentration was 228.0 ± 032.80 ppm. The difference in mean salivary fluoride concentration was statistically significant (p < 0.001), indicating that chewing sticks are a cost-effective and efficient means of caries prevention. Although multiple pharmacological effects are assigned to A. laxiflora, a smaller number of clinical trials have been conducted. Hence, more clinical studies are required to establish the pharmacological significance or clinical applications of A. laxiflora.
Other applications
The ethanol extract of A. laxiflora leaves was evaluated against a field to store pests of maize grains, namely, maize weevil (Sitophilus zeamais). The extract displayed significant insecticidal activity in stored maize seeds, suggesting an eco-friendly alternative to synthetic pesticides (Ileke et al., 2020) (Figure 5). The A. laxiflora leave extract has been reported to prevent the corrosion of mild steel in an acidic medium (Olasehinde et al., 2015). The A. laxiflora stem bark and leave extract has been used as a reducing and capping agent for synthesizing platinum and bimetallic platinum-copper nanoparticles for catalytic oxidative desulfurization of model oil (Olajire et al., 2018; 2017b; 2017a). Similarly, Ekennia et al. (2021) biosynthesized quasi-hexagonal zinc oxide nanoparticles using the aqueous leave extract. Moreover, the synthesized nanoparticles exhibited good photocatalytic activity against Congo red dye solution and mushroom tyrosinase inhibitory activity.
Conclusion
This review highlights crucial information about the traditional use, phytochemistry, and pharmacological activities of crude extracts, as well as the phytochemicals of A. laxiflora. Diverse ethnomedicinal uses are linked with various parts of A. laxiflora, including the treatment of malaria, diabetes, sickle cell anemia, inflammatory conditions, skin disorders, and venereal diseases; gastrointestinal problems such as hepatitis, stomachache, dysentery, and piles; and neurological disorders such as anxiety, depression, insomnia, and epilepsy. Although several pharmacological activities of A laxiflora have been reported based on ethnomedicine uses, some must be validated pharmacologically. Meanwhile, most pharmacological activities have been reported using crude extracts rather than isolated compounds. As a result, further research is required to determine the links between isolated chemicals and medicinal uses. Phytochemical investigations revealed the presence of alkaloids, flavonoids, terpenoids, phenolic compounds, and fatty acids. The compounds 3-acetyloleanolic acid, 3-acetoxyursolic acid, ellagic acid, and quercetin and their derivatives could be drug candidates for treating HIV, cancer, and microbial infections because of their potent biological activities. This study suggests that in-depth investigations on the mechanism of action, the pharmacological activity of isolated compounds, and toxicological analysis of biologically active extracts and active compounds of A. laxiflora are all worthwhile endeavors. Research in these areas could support possible medicinal uses and future development into therapeutic modalities.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors without undue reservation.
Author contributions
NJ and BC planned the review outline, content, and structure. NJ, MT, SK, BC, YA, and PW wrote the manuscript and created the figures. HC, AK, HD, MB, and KC contributed to editing the manuscript, tables, and figures. All authors approve the manuscript for publication.
Funding
This research was funded by the Deanship of Scientific Research at King Khalid University, Abha, Saudi Arabia, Grant Number R.G.P.2/27/40. The authors are grateful to the authorities of ITM University for providing the necessary resources.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.958453/full#supplementary-material
References
Adebisi, M. A. (2019). Ethnobotany survey of medicinal plants used in the treatment of fibroid in Ogun and Osun states, southwestern, Nigeria. J. Res. For. Wildl. Environ. 11, 33–44.
Adeloye, A. O., Aderogba, M. A., Idowu, T. O., Obuotor, E. M., and Ogundaini, A. O. (2005). Investigation of the antioxidant activity of alchornea laxiflora (benth) and its constituents. J. Food Technol. 3, 365–369.
Adeniran, M. A. (2015). Ethnobotanical treatize on selected herbs used in the treatment of hypertension among the Ekiti people of western Nigeria. Int. J. Heal. Med. Inf. 4, 23–27.
Adeniran, M. A., and Falemu, F. A. (2017). Plants used as anti-anaemic and haematinic agents among indigenes in ijero local government area , Ekiti state , Nigeria. J. Environ. Issues Agric. Dev. Ctries. 9, 9–15.
Agbo, M. O., Okoye, F. B. C., Ebi, G. C., and Osadebe, P. O. (2020). <i>Alchornea floribunda</i> (Müll. Arg.) - a review of its phytochemistry and biological activities. Trop. J. Pharm. Res. 19, 1113–1120. doi:10.4314/tjpr.v19i5.30
Ajibesin, K. K., Ekpo, B. A., Bala, D. N., Essien, E. E., and Adesanya, S. A. (2007). Ethnobotanical survey of akwa ibom state of Nigeria. J. Ethnopharmacol. 115, 387–408. doi:10.1016/j.jep.2007.10.021
Akinpelu, D. A., Abioye, E. O., Aiyegoro, O. A., Akinpelu, O. F., and Okoh, A. I. (2015). Evaluation of antibacterial and antifungal properties of alchornea laxiflora (Benth.) Pax. & Hoffman. Evidence-based Complement. Evid. Based. Complement. Altern. Med. 2015, 684839. doi:10.1155/2015/684839
Ali, S. I., Gopalakrishnan, B., and Venkatesalu, V. (2017). Pharmacognosy, phytochemistry and pharmacological properties of Achillea millefolium L. A review. Phytother. Res. 31, 1140–1161. doi:10.1002/ptr.5840
Amujoyegbe, O. O., Idu, M., Agbedahunsi, J. M., and Erhabor, J. O. (2016). Ethnomedicinal survey of medicinal plants used in the management of sickle cell disorder in Southern Nigeria. J. Ethnopharmacol. 185, 347–360. doi:10.1016/j.jep.2016.03.042
Aweto, A. O. (2001). Trees in shifting and continuous cultivation farms in Ibadan area, southwestern Nigeria. Landsc. Urban Plan. 53, 163–171. doi:10.1016/S0169-2046(00)00151-1
Bada, S., Oyetayo, A., Adaramola-Ajibola, K., and Giwa, E. (2017). A preliminary study of the effect of Alchornea laxiflora on haematological parameters and organ weight in albino rats. Int. Blood Res. Rev. 7, 1–6. doi:10.9734/ibrr/2017/35722
Bafor, E. E., Eyohan, S. E., Omoruyi, O., Elvis-Offiah, U. B., Ayinde, B., Eze, G. I., et al. (2015). Preliminary endocrinological, histological and haematological investigation of Alchornea laxiflora (Euphorbiaceae) leaf extract effects on the ovary, uterus and cervix of mouse models. J. Sci. Pr. Pharm. Dec. 2, 55–63.
Bafor, E. E., Nwogu, J. K., Elvis-Offiah, U. B., Amaechina, F., Ofeimun, J., Ayinde, B., et al. (2018). Modulation of ex-vivo uterine contraction by the methanol leaf extract of Alchornea laxiflora Benth. (Euphorbiaceae) and preliminary spectrometric identification of associated secondary metabolites. J. Med. Plants Econ. Dev. 2, 1–13. doi:10.4102/jomped.v2i1.33
Bamimore, V. O., and Elujoba, A. A. (2018). Antisickling properties of three medicinal plants and their combinations. Int. J. Pharmacogn. 5, 666–672. doi:10.13040/IJPSR.0975-8232
Boniface, P. K., Ferreira, S. B., and Kaiser, C. R. (2016). Recent trends in phytochemistry, ethnobotany and pharmacological significance of Alchornea cordifolia. J. Ethnopharmacol. 191, 216–244. doi:10.1016/j.jep.2016.06.021
Borokini, T. I., Clement, M., Dickson, N. J., and Edagbo, D. E. (2013). Ethnobiological survey of traditional medicine practice for circulatory and nervous system related diseases in Oyo State. Topclass J. Herb. Med. 2, 111–120.
Bueno, J. (2012). In vitro antimicrobial activity of natural products using minimum inhibitory concentrations: Looking for new chemical entities or predicting clinical response. Med. Aromat. Plants 01, 1–2. doi:10.4172/2167-0412.1000113
Bum, E. N., Taiwe, G. S., Nkainsa, L. A., Moto, F. C. O., Seke Etet, P. F., Hiana, I. R., et al. (2009). Validation of anticonvulsant and sedative activity of six medicinal plants. Epilepsy Behav. 14, 454–458. doi:10.1016/j.yebeh.2008.12.022
Dzoyem, J. P., and Eloff, J. N. (2015). Anti-inflammatory, anticholinesterase and antioxidant activity of leaf extracts of twelve plants used traditionally to alleviate pain and inflammation in South Africa. J. Ethnopharmacol. 160, 194–201. doi:10.1016/j.jep.2014.11.034
Ekennia, A., Uduagwu, D., Olowu, O., Nwanji, O., Oje, O., Daniel, B., et al. (2021). Biosynthesis of zinc oxide nanoparticles using leaf extracts of Alchornea laxiflora and its tyrosinase inhibition and catalytic studies. Micron 141, 102964. doi:10.1016/J.MICRON.2020.102964
Eloff, J. N. (2019). Avoiding pitfalls in determining antimicrobial activity of plant extracts and publishing the results. BMC Complement. Altern. Med. 19, 106. doi:10.1186/s12906-019-2519-3
Elufioye, T. O. (2017). “Memory-enhancing and memory-related beneficial effects of selected medicinal plants from the Nigerian flora,” in Medicinal plants and fungi: Recent advances in research and development. Editors D. Agrawal, H. S. Tsay, L. F. Shyur, Y. C. Wu, and S. Y. Wang (Singapore: Springer), 487–510. doi:10.1007/978-981-10-5978-0_15
Emeke, U., Obontu, T. J., Olushola, I., and Akinyele, A. (2019). Salivary fluoride retention: A comparative analysis between fluoride containing chewing sticks and a non herbal fluoridated toothpaste. J. Contemp. Dent. Pract. 20, 370–376. doi:10.5005/jp-journals-10024-2524
Esosa, U. S., Kingsley, O., Georgina, E. O., Sunday, J. J., and Spencer, N. C. O. (2013). Possible reversal of sodium arsenate-induced liver toxicity by hexane leaf extract of Alchornea laxiflora. Asian J. Med. Sci. 5, 03–08. doi:10.19026/ajms.5.5339
Essiett, A., and Ajibesin, K. (2010). Antimicrobial activities of some euphorbiaceae plants used in the traditional medicine of akwa ibom state of Nigeria. Ethnobot. Leafl. 14, 654–664.
Fabeku, P. O., and Akinsulire, O. (2008). “The role of traditional medicine among the Yoruba tribe of South-west Nigeria,” in A textbook of medicinal plants from Nigeria. Editor T. Odugbemi (Lagos: University of Lagos press), 43–54.
Farombi, E. O., Ogundipe, O. O., Uhunwangho, E. S., Adeyanju, M. A., and Moody, J. O. (2003). Antioxidant properties of extracts from Alchornea laxiflora (benth) Pax and hoffman. Phytother. Res. 17, 713–716. doi:10.1002/ptr.1050
Firenzuoli, F., and Gori, L. (2007). Herbal medicine today: Clinical and research issues. Evid. Based. Complement. Altern. Med. 4, 37–40. doi:10.1093/ecam/nem096
Fongod, A. G. N., Ngoh, L. M., and Veranso, M. C. (2014). Ethnobotany, indigenous knowledge and unconscious preservation of the environment: An evaluation of indigenous knowledge in South and Southwest Regions of Cameroon. Int. J. Biodivers. Conserv. 6, 85–99. doi:10.5897/ijbc2013.0637
Gbadamosi, I. (2015). An inventory of ethnobotanicals used in the management of sickle cell disease in oyo state , Nigeria an inventory of ethnobotanicals used in the management of sickle cell disease in oyo state , Nigeria. Bot. Res. Int. 8, 65–72. doi:10.5829/idosi.bri.2015.8.4.523
Geethangili, M., and Ding, S. T. (2018). A review of the phytochemistry and pharmacology of Phyllanthus urinaria L. Front. Pharmacol. 9, 1109–1120. doi:10.3389/fphar.2018.01109
Heinrich, M., Appendino, G., Efferth, T., Fürst, R., Izzo, A. A., and Kayser, O. (2020). Best practice in research overcoming common challenges in phytopharmacological research. J. Ethnopharmacol. 246, 112230. doi:10.1016/j.jep.2019.112230
Höft, R., and Höft, M. (1995). The differential effects of elephants on rain forest communities in the Shimba Hills, Kenya. Biol. Conserv. 73, 67–79. doi:10.1016/0006-3207(95)90067-5
Hossain, M. S., Urbi, Z., Sule, A., and Rahman, K. M. H. (2014). Andrographis paniculata (burm. F.) wall. Ex nees: A review of ethnobotany, phytochemistry, and pharmacology. ScientificWorldJournal. 2014, 274905. doi:10.1155/2014/274905
Hutchinson, J., and Dalziel, J. M., 1937. Tropical african plants: XVI. Bull. Misc. Inf. 1937, 333–341. doi: doi:10.2307/4107186
Ihejirika, G. O., Nwufo, M. I., Ibeawuchi, I. I., Obilo, O. P., Ofor, M. O., Ogbedeh, K. O., et al. (2015). Effect of processing and packaging materials on the storability and microorganisms associated with Garcinia kola (bitter kola). Agric. For. Fish. 4, 51–58. doi:10.11648/j.aff.s.2015040301.19
Ileke, K. D., Idoko, J. E., Ojo, D. O., and Adesina, B. C. (2020). Evaluation of botanical powders and extracts from Nigerian plants as protectants of maize grains against maize weevil, Sitophilus zeamais (motschulsky) [Coleoptera: Curculionidae]. Biocatal. Agric. Biotechnol. 27, 101702. doi:10.1016/j.bcab.2020.101702
Jayeoba, O. J., Ijeomah, H. M., and Ogara, I. M. (2012). Ethnomedical utilization of Alchornea laxiflora (benth) Pax & K Hoffm in Irepodun/Ifelodun local government area of Ekiti state, Southwest, Nigeria. J. Agric. Soc. Res. 12, 32.
Kabuo, N. O., Uzuegbu, J. O., Ubbaonu, C. N., and Onyeka, E. U. (2013). The microorganisms and compounds influencing the organoleptic properties of Ugba (fermented Pentaclethra macrophylla Benth. seeds). Afr. J. Food Sci. 7, 25–34. doi:10.5897/ajfs11.195
Kaur, R., Kumar, S., and Sharma, A. K. (2012). Medicinal plants for the treatment of sexual transmitted diseases. Int. J. Pharm. Innov. 2, 13–19.
Kuete, V., Tchinda, C. F., Mambe, F. T., Beng, V. P., and Efferth, T. (2016). Cytotoxicity of methanol extracts of 10 Cameroonian medicinal plants towards multi-factorial drug-resistant cancer cell lines. BMC Complement. Altern. Med. 16, 267. doi:10.1186/s12906-016-1253-3
Magwede, K., van Wyk, B. E., and van Wyk, A. E. (2019). An inventory of Vhavenḓa useful plants. S. Afr. J. Bot. 122, 57–89. doi:10.1016/j.sajb.2017.12.013
Mahomoodally, M. F. (2013). Traditional medicines in Africa: An appraisal of ten potent african medicinal plants. Evid. Based. Complement. Altern. Med. 2013, 617459. doi:10.1155/2013/617459
Makinde, S. C. O., Ojekale, A. B., Oshinaike, T. S., and Awusinu, T. S. (2015). An ethnomedical and ethnobotanical survey of plants herbal therapy used for obesity, asthma, diabetes and fertility by the Badagry people of lagos state, Nigeria. J. Med. Plants Stud. 3, 01–06.
Maroyi, A. (2016). A review of ethnoboatany, therapeutic value, phytochemistry and pharmacology of Crinum macowanii baker: A highly traded bulbous plant in southern Africa. J. Ethnopharmacol. 194, 595–608. doi:10.1016/j.jep.2016.10.046
Martínez, C. A., Mosquera, O. M., and Niño, J. (2017). Medicinal plants from the genus alchornea (euphorbiaceae): A review of their ethnopharmacology uses and phytochemistry. Bol. Latinoam. del Caribe Plantas Aromat. 16, 162–205.
Matuschek, E., Brown, D. F. J., and Kahlmeter, G. (2014). Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin. Microbiol. Infect. 20, O255–O266. doi:10.1111/1469-0691.12373
Mbaveng, A. T., Sandjo, L. P., Tankeo, S. B., Ndifor, A. R., Pantaleon, A., Nagdjui, B. T., et al. (2015). Antibacterial activity of nineteen selected natural products against multi-drug resistant Gram-negative phenotypes. Springerplus 4, 823. doi:10.1186/s40064-015-1645-8
McCarthy, M. S., Lester, J. D., and Stanford, C. B. (2017). Chimpanzees (Pan troglodytes) flexibly use introduced species for nesting and bark feeding in a human-dominated habitat. Int. J. Primatol. 38, 321–337. doi:10.1007/s10764-016-9916-y
Molander, M., Nielsen, L., Søgaard, S., Staerk, D., Rønsted, N., Diallo, D., et al. (2014). Hyaluronidase, phospholipase A2 and protease inhibitory activity of plants used in traditional treatment of snakebite-induced tissue necrosis in Mali, DR Congo and South Africa. J. Ethnopharmacol. 157, 171–180. doi:10.1016/j.jep.2014.09.027
Morah, F. N. I., and Uduagwu, D. N. (2017). Chemical composition , antioxidant and larvicidal activity of Alchornea laxiflora ( Benth ) leaf extracts. Edorium J. Pharmacol. 1, 1–8. doi:10.5348/P06-2017-1-OA-1
Moundipa, P. F., Flore, K. G. M., Bilong Bilong, C. F., and Bruchhaus, I. (2005). <i>in vitro</i> Amoebicidal Activity of some Medicinal Plants of the Bamun Region ( Cameroon). Afr. J. Tradit. Complement. Altern. Med. 2, 113–121. doi:10.4314/ajtcam.v2i2.31109
Muller, T., Sitoe, A., and Mabunda, R., 2005. Assessment of the forest reserve network in Mozambique, Unpublished report.
Mwavu, E. N., and Witkowski, E. T. F. (2008). Sprouting of woody species following cutting and tree-fall in a lowland semi-deciduous tropical rainforest, North-Western Uganda. For. Ecol. Manage. 255, 982–992. doi:10.1016/j.foreco.2007.10.018
Nabatanzi, A., Nkadimeng, S. M., Lall, N., Kabasa, J. D., and McGaw, L. J. (2020). Ethnobotany, phytochemistry and pharmacological activity of kigelia africana (Lam.) benth. (bignoniaceae). Plants 9, 753. doi:10.3390/plants9060753
Ndam Ngoungoure, V. L., Mfotie Njoya, E., Ngamli Fewou, S., Fils Ella, A., McGaw, L. J., and Moundipa, P. F. (2019). Acetylcholinesterase inhibitory, anti-inflammatory and antioxidant properties of some Cameroonian medicinal plants used to treat some neurological disorders. Invest. Med. Chem. Pharmacol. 2, 1–13. doi:10.31183/imcp.2019.00033
Ngnameko, C. R., Njayou, F. N., Fowora, M., Nemg, F. B. S., Moundipa Fewou, P., and Smith, S. I. (2019). Inhibitory effect of medicinal plants from Cameroon on the growth and adhesion of Helicobacter pylori. Eur. J. Integr. Med. 30, 100957. doi:10.1016/j.eujim.2019.100957
Ngoungoure, V. L. N., Muñoz, P., Tizabi, Y., Valdes, R., Moundipa, P. F., and Segura-Aguilar, J. (2019). Protective effects of crude plant extracts against aminochrome-induced toxicity in human astrocytoma cells: Implications for Parkinson’s disease. Clin. Pharmacol. Transl. Med. 3, 125–133.
Nimenibo-uadia, R. (2018). Antihyperglycaemic effect of methanol leaf extract of Alchornea laxiflora ( benth ) Pax and hoffman in diabetic rats. J. Nat. Sci. Res. 8, 4–10.
Njamen, D., Mvondo, M. A., Djiogue, S., Wanda, G. J. M. K., Nde, C. B. M., and Vollmer, G. (2013). Phytotherapy and Womenʼs reproductive health: The Cameroonian perspective. Planta Med. 79, 600–611. doi:10.1055/s-0032-1328326
Njayou, F., Moundipa, P., Tchana, A., Ngadjui, B., and Tchouanguep, F. (2008). Inhibition of microsomal lipid peroxidation and protein oxidation by extracts from plants used in Bamun folk medicine (Cameroon) against hepatitis. Afr. J. Tradit. Complement. Altern. Med. 5, 278–289. doi:10.4314/ajtcam.v5i3.31284
Nwonu, C., Ilesanmi, O., Agbedahunsi, J., and Nwonu, P. (2018a). Analgesic profile of the aqueous and methanol extracts of alchornea laxiflora in albino mice. Eur. Sci. J. ESJ 14, 134. doi:10.19044/esj.2018.v14n24p134
Nwonu, C., Ilesanmi, O., Agbedahunsi, J., and Nwonu, P. (2018b). Anti-anxiety activities of the aqueous and methanol extracts of Alchornea laxiflora in albino mice. Sci. Res. J. 6, 69–77. doi:10.29322/ijsrp.8.5.2018.p7781
Nwonu, C., Ilesanmi, O., Agbedahunsi, J., and Nwonu, P. (2018c). Effects of the aqueous and methanol extracts of Alchornea laxiflora in rodent models of experimental psychosis. IOSR J. Pharm. Biol. Sci. 13, 46–52. doi:10.9790/3008-1304064652
Obodai, E., and Nsor, C. (2009). Aspects of biodiversity and fish production in the kukobila wetland in the savelugu- nanton district of the northern region, Ghana. Ethiop. J. Env. Stud. Manag. 2. doi:10.4314/ejesm.v2i3.48266
Ochieng, M. A., Ben Bakrim, W., Bitchagno, G. T. M., Mahmoud, M. F., and Sobeh, M. (2022). Syzygium jambos L. Alston: An insight into its phytochemistry, traditional uses, and pharmacological properties. Front. Pharmacol. 13, 786712–786715. doi:10.3389/fphar.2022.786712
Odugbemi, T., and Akinsulire, O. (2008). “Medicinal plants species, family names and uses,” in A textbook of medicinal plants from Nigeria. Editor T. Odugbemi (Lagos: University of Lagos press), 542–564.
Ogbole, O. O., Aliu, L. O., Abiodun, O. O., and Ajaiyeoba, E. O. (2016). Alpha-amylase inhibition and brine shrimp lethality activities of nine medicinal plant extracts from south-west Nigerian ethnomedicine. J. Herbs, Spices Med. Plants 22, 319–326. doi:10.1080/10496475.2016.1214941
Ogundipe, O. O., Moody, J. O., and Houghton, P. J. (2001a). Occurrence of flavonol sulphates in Alchornea laxiflora. Pharm. Biol. 39, 421–423. doi:10.1076/phbi.39.6.421.5888
Ogundipe, O. O., Moody, J. O., Houghton, P. J., and Odelola, H. A. (2001b). Bioactive chemical constituents from Alchornea laxiflora (benth) Pax and Hoffman. J. Ethnopharmacol. 74, 275–280. doi:10.1016/S0378-8741(00)00352-4
Okaiyeto, K., and Oguntibeju, O. O. (2021). African herbal medicines : Adverse effects and cytotoxic potentials with different therapeutic applications. Int. J. Environ. Res. Public Health 18, 5988. doi:10.3390/ijerph18115988
Okokon, J. E., Augustine, N. B., and Mohanakrishnan, D. (2017a). Antimalarial, antiplasmodial and analgesic activities of root extract of Alchornea laxiflora. Pharm. Biol. 55, 1022–1031. doi:10.1080/13880209.2017.1285947
Okokon, J. E., Obot, A. U., Mohanakrishnan, D., Mittal, G., and Sahal, D. (2017b). Antimalarial and antiplasmodial activity of leaf extract of Alchornea laxiflora. J. Herbs, Spices Med. Plants 23, 128–141. doi:10.1080/10496475.2016.1275072
Oladiji, A. T., Olatunde, A., and Oloyede, H. O. B. (2014). Evaluation of Anti-anaemic potential of aqueous extract of Alchornea laxiflora (Benth) leaf in iron deficient Rats. Acad. Arena 6, 22–29.
Oladunmoye, M. K., and Kehinde, F. Y. (2011). Ethnobotanical survey of medicinal plants used in treating viral infections among Yoruba tribe of South Western Nigeria. Afr. J. Microbiol. Res. 5, 2991–3004. doi:10.5897/AJMR10.004
Olajire, A. A., Adeyeye, G. O., and Yusuf, R. A. (2017a). Alchornea laxiflora bark extract assisted green synthesis of platinum nanoparticles for oxidative desulphurization of model oil. J. Clust. Sci. 28, 1565–1578. doi:10.1007/s10876-017-1167-3
Olajire, A. A., Ifediora, N. F., Bello, M. D., and Benson, N. U. (2018). Green synthesis of copper nanoparticles using Alchornea laxiflora leaf extract and their catalytic application for oxidative desulphurization of model oil. Iran. Iran. J. Sci. Technol. Trans. Sci. 42, 1935–1946. doi:10.1007/s40995-017-0404-9
Olajire, A. A., Kareem, A., and Olaleke, A. (2017b). Green synthesis of bimetallic Pt@Cu nanostructures for catalytic oxidative desulfurization of model oil. J. Nanostructure Chem. 7, 159–170. doi:10.1007/s40097-017-0223-8
Olanipekun, M. K., and Aladetimiro, B. O. (2017). Cheklist of plants used traditionally to treat menstrual disorders in ekiti-state, Nigeria. Need for conservation as a sustainable practice in healthcare management in rural areas. IOSR J. Pharm. Biol. Sci. 12, 10–16. doi:10.9790/3008-1201041016
Olanipekun, M. K., Arowosegbe, S., Kayode, J. O., and Oluwole, T. R. (2016). Ethnobotanical survey of medicinal plants used in the treatment of women related diseases in Akoko Region of Ondo-State, Nigeria. J. Med. Plants Res. 10, 270–277. doi:10.5897/JMPR2015.6040
Olasehinde, E. F., Ogunjobi, J. K., and Akinlosotu, O. M. (2015). Investigation of the inhibitive properties of Alchornea laxiflora leaves on the corrosion of mild steel in HCl: Thermodynamics and kinetic study. J. Am. Sci. 11, 1545–1003.
Oloyede, G. K., Onocha, P. A., Adaramoye, O. A., and Thonda, S. E. (2011). Hepatoprotective activity and flavonoids of Alchornea laxiflora leaf extract. Res. J. Phytochemistry 5, 190–200. doi:10.3923/rjphyto.2011.190.200
Oloyede, G. K., Onocha, P. A., Soyinka, J., Oguntokun, O. W., and Thonda, E. (2010). Phytochemical screening, antimicrobial and antioxidant activities of four Nigerian medicinal plants. Ann. Biol. Res. 1, 114–120.
Oluyemi, O. F., and Blessing, O. A. (2019). Prophylactic and curative potency of Alchornea laxiflora extract on Plasmodium berghei infected Swiss albino mice. Int. J. Trop. Dis. Health 36, 1–11. doi:10.9734/ijtdh/2019/v36i130134
Osabiya, O., Bada, S., Ibitoye, F., and Aribisala, O. (2017). Assessment of phytochemical constituent, antibacterial and cytotoxic activities of A. laxiflora (Ewe pepe) extracts. J. Appl. Life Sci. Int. 12, 1–9. doi:10.9734/jalsi/2017/34038
Osuntokun, O., and Olajubu, F. (2015). Antibacterial and phytochemical properties of some Nigerian medicinal plants on Salmonella typhi and Salmonella paratyphi isolated from human stool in owo local government, ondo state , Nigeria. J. Sci. Res. Rep. 4, 441–449. doi:10.9734/jsrr/2015/12021
Otuechere, C., Durugbo, E. U., and Osho, A. (2019). Essential oil of Alchornea laxiflora (benth): Phytochemical, antimicrobial and toxicity evaluations. Lett. Appl. NanoBioScience 8, 661–665. doi:10.33263/lianbs83.661665
Oyeyemi, I. T., Akinseye, K. M., Adebayo, S. S., Oyetunji, M. T., and Oyeyemi, O. T. (2019). Ethnobotanical survey of the plants used for the management of malaria in Ondo State, Nigeria. S. Afr. J. Bot. 124, 391–401. doi:10.1016/j.sajb.2019.06.003
Phumthum, M., Balslev, H., and Barfod, A. S. (2019). Important medicinal plant families in Thailand. Front. Pharmacol. 10, 1125. doi:10.3389/fphar.2019.01125
Rivera, D., Allkin, R., Obón, C., Alcaraz, F., Verpoorte, R., and Heinrich, M. (2014). What is in a name? The need for accurate scientific nomenclature for plants. J. Ethnopharmacol. 152, 393–402. doi:10.1016/j.jep.2013.12.022
Sandjo, L. P., Poumale, H. M. P., Siwe, X. N., Ntede, H. N., Shiono, Y., Ngadjui, B. T., et al. (2011). Two new fatty acid derivatives from the stem bark of Alchornea laxiflora (euphorbiaceae). J. Americ. Oil Chem. Soc. 88, 1153–1159. doi:10.1007/s11746-011-1770-7
Simpson, M. G. (2010). “Diversity and classification of flowering plants: Eudicots,” in Plant Syst., 275–448. doi:10.1016/B978-0-12-374380-0.50008-7
Siwe-Noundou, X., Ndinteh, D. T., Olivier, D. K., Mnkandhla, D., Isaacs, M., Muganza, F. M., et al. (2019). Biological activity of plant extracts and isolated compounds from Alchornea laxiflora: Anti-HIV, antibacterial and cytotoxicity evaluation. S. Afr. J. Bot. 122, 498–503. doi:10.1016/j.sajb.2018.08.010
Soladoye, M. O., Chukwuma, E. C., Sulaiman, O. M., and Feyisola, R. T. (2014). Ethnobotanical survey of plants used in the traditional treatment of female infertility in southwestern Nigeria. Ethnobot. Res. Appl. 12, 81–90. doi:10.17348/era.12.0.081-090
Tapondjou, L. A., Jeuett-Siems, K., Siems, K., and Melzig, M. F. (2016). Alchornealaxine, an unusual prenylguanidinyl-epicatechin derivative from Alchornea laxiflora (benth) Pax and hoffman. Rec. Nat. Prod. 10, 508–512.
Tchinda, C. F., Voukeng, I. K., Beng, V. P., and Kuete, V. (2017). Antibacterial activities of the methanol extracts of Albizia adianthifolia, Alchornea laxiflora, Laportea ovalifolia and three other Cameroonian plants against multi-drug resistant Gram-negative bacteria. Saudi J. Biol. Sci. 24, 950–955. doi:10.1016/j.sjbs.2016.01.033
Tegene, A. S. (2018). Vegetation types , composition and conservation status of forest ecosystems in Ethiopia. J. Nat. Sci. Res. 8, 20–30.
Ugbogu, O. A., and Chukwuma, E. C. (2019). Ethnobotany of okomu forest reserve, Edo state, Nigeria. J. Appl. Sci. Environ. Manag. 23, 1391. doi:10.4314/jasem.v23i7.31
Uhunmwangho, E., Rasaq, N., and Ojemekele, O. (2016). Protective effect of Alchornea laxiflora methanolic leaf extract on CCL4-induced hepatotoxicity and reproductive toxicity in male wistar rats. Br. J. Pharm. Res. 14, 1–7. doi:10.9734/bjpr/2016/29807
Uhunmwangho, E., Ojemekele, O., and Salemcity, A. (2017). Effects of methanol leaf extract of Alchornea laxiflora on antioxidant enzymes , triacylglycerol and cholesterol levels in male wistar rats. Int. J. Life Sci. Res. 5, 93–97.
Uhunmwangho, E. S., Rasaq, N. O., and Osikoya, I. O. (2018). Hepatoprotective effects of hexane root extract of Alchornea laxiflora in sodium arsenate toxicity in wistar albino rats. CHRISMED J. Health Res. 5, 38–42. doi:10.4103/cjhr.cjhr_33_17
Verlhac, L., Izumi, K., Lézine, A. M., Lemonnier, K., Buchet, G., Achoundong, G., et al. (2018). Altitudinal distribution of pollen, plants and biomes in the Cameroon highlands. Rev. Palaeobot. Palynol. 259, 21–28. doi:10.1016/j.revpalbo.2018.09.011
Wansi, S. L., Ruphin, C., Deumeni, M., Laure, S., Kamani, P., Sama, L. F., et al. (2017). Antidiarrhoeal activity of aqueous and methanolic Alchornea laxiflora (Euphorbiaceae) leaves extracts in rats. J. Med. Plants Stud. 5, 205–211.
Glossary
AGS Accelerated gradient chromatography
MS Mass spectrometry
NMR Nuclear magnetic resonance
SLHC Sephadex-20 column chromatography
HR-ESI-TOF-MS High resolution-electrospray ionization-time of flight-mass spectrometry
GC-MS Gas chromatography-mass spectrometry
ZOI Zones of inhibition
INT Iodonitrotetrazolium
p.o. per oral
s.c. Subcutaneous
MES Maximal electroshock
PTZ Pentylenetetrazol
NMDA N-Methyl-D-aspartate
INH Isonicotinic hydrazide acid
PIC Picrotoxin
STR Strychnine
HIV Human immunodeficiency virus
HeLa cells human cervix adenocarcinoma
IC50 Half maximal inhibitory concentration
ASA Amino salicylic acid
15-LOX 15-Lipoxygenase
NO Nitric oxide
LPS Bacterial lipopolysaccharides
MIC Minimum inhibitory concentration
MBC Minimum bactericidal concentration
CQ Chloroquine
CCl4 Carbon tetrachloride
ALT Alanine aminotransferase
AST Aspartate aminotransferase
ALP Alkaline phosphatase
LDH Lactate dehydrogenase
GGT Gamma-glutamyl transferase
GST Glutathione-s-transferase
NAD 4-Nitroanisole demethylase
PCV Packed cell volume
Hb Hemoglobin
RBC Red blood cell
MCV Mean corpuscular volume
MCH Mean corpuscular hemoglobin
MCHC Mean corpuscular hemoglobin concentration
ACh Acetylcholine
AChE Acetylcholine esterase
BuChE Butylcholine esterase
ABTS 2,2 Azinobis-(3-ethylbenzothiazoline-6-sulfonic acid)
BHA Butylated hydroxyanisole
DPPH 2, 2-Diphenyl-1-picrylhydrazyl
FTC Ferric thiocyanate
FRAP Ferric reducing antioxidant power
CAT Catalase
SOD Superoxide dismutase
GSH Reduced glutathione
EC50 Half maximal effective concentration
CCRF-CEM Human T lymphoblast cell line
HEK Human embryonic kidney cells
HL60 Human promyelocytic leukemia
MTT 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
TC50 Median toxic concentration
LD50 Median lethal dose
KCl Potassium chloride
Keywords: Alchornea laxiflora, ethnopharmacology, African plants, traditional medicine, phytochemistry, pharmacology
Citation: Jain NK, Tailang M, Kumar S, Chandrasekaran B, Alghazwani Y, Chandramoorthy HC, Kumar A, Deshpande H, Wal P, Balamurugan M and Chidambaram K (2022) Appraising the therapeutical potentials of Alchornea laxiflora (Benth.) Pax & K. Hoffm., an underexplored medicinal herb: A systematic review. Front. Pharmacol. 13:958453. doi: 10.3389/fphar.2022.958453
Received: 31 May 2022; Accepted: 31 October 2022;
Published: 02 December 2022.
Edited by:
Abdelhakim Bouyahya, Mohammed V University, MoroccoReviewed by:
Smith B. Babiaka, University of Buea, CameroonSuman Chowdhury, The State University of New Jersey, United States
Copyright © 2022 Jain, Tailang, Kumar, Chandrasekaran, Alghazwani, Chandramoorthy, Kumar, Deshpande, Wal, Balamurugan and Chidambaram. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Balakumar Chandrasekaran, dhillbalu@gmail.com; Kumarappan Chidambaram, kumarappan@kku.edu.sa
 Nem Kumar Jain
Nem Kumar Jain Mukul Tailang
Mukul Tailang Santosh Kumar3
Santosh Kumar3 Balakumar Chandrasekaran
Balakumar Chandrasekaran Kumarappan Chidambaram
Kumarappan Chidambaram