Introduction
Materials and Methods
Seed Materials
Dormancy Classification Method
Seed Germination Conditions
Chemical treatments
Investigations and Statistical Analysis
Results
Classification of Seed Dormancy Types on Well-selected Seeds
Seed Germination Conditions
Effects of Chemical Treatments on Seed Germination
Discussion
Introduction
Many wild plants native to the Republic of Korea are currently being actively studied for their functionality, edibility, and ornamental values for development as horticultural crops. Astilboides tabularis is a perennial herb of the Saxifragaceae family that is found in northern Gyeonggi-do and around Gangwon-do in Korea. This plant, which is usually found underneath trees in deep ravines, is approximately 1 m in length. The stem is straight and piliferous. A. tabularis is a rosette plant with short petioles and the middle leaf separated into approximately seven parts. Particularly large leaves are approximately 75 cm in diameter and serrated. The panicles are white and neutral, and the plant blooms in June or July. The fruit is a capsule (Lee, 2003).
The tender leaves of A. tabularis are edible, and all aboveground plant parts can be used medicinally. In addition, the rhizome is a good source of tannin (Food Materials Information, 2015). Therefore, A. tabularis has strong potential for use as a functional food and herbal source. In addition, this plant can be grown in the shade and has large, green leaves with ornamental value, suggesting that it can be used as landscaping material, particularly under tall trees. Nevertheless, A. tabularis is one of the specific plant species of the floral region (Ministry of Environment, 2007) and is also an endangered species in Korea (Ministry of Environment, 2012). While propagation research for A. tabularis is urgently needed, no such research has been conducted.
Dormancy, the plant’s process of recognizing environmental suitability for germination, differs according to species and growth condition (Baskin and Baskin, 1998; Geneve, 2003). Dormancy types vary depending on the researcher, and each type has been further subcategorized. Each dormancy type requires different breaking methods (Baskin and Baskin, 2004b). Little research has been performed on dormancy type for native plant seeds in Korea; such work has been limited to improving germination rates by low temperature and growth regulator treatments (Kang et al., 2014; Lee et al., 2014a; 2014b).
The current study was conducted to develop an effective seed propagation method for uniform seedling production in A. tabularis by analyzing the seed dormancy types and germination characteristics of this plant.
Materials and Methods
Seed Materials
A. tabularis seeds were collected around Gangwon-do in July of 2012, cleaned to remove impurities, and dried indoors for 24 hours. Well-selected seeds were placed into a sealed zipper bag with silica gels (Mr. Keeper, Sungel, Korea) and stored in a low-temperature warehouse at 4 ± 1.0°C during the experiments. The length, width, and 1,000-seed weight were measured using digital Vernier calipers (NA500-150S, Bluebird, China) and an electronic scale (IB-610S, Innotem, Incheon, Korea). Moisture content was measured based on the difference in weight before and after the seeds were dried at 70°C with hot air for 48 hours.
Dormancy Classification Method
Baskin and Baskin (2004a, 2004b) and Nicolaeva (2001) were referenced for classification of seed dormancy type. The final analyses were based on (among other methods) embryo observations in seeds, immediate sowing upon collection, and detecting any difficulties with the seed coat in terms of moisture absorption.
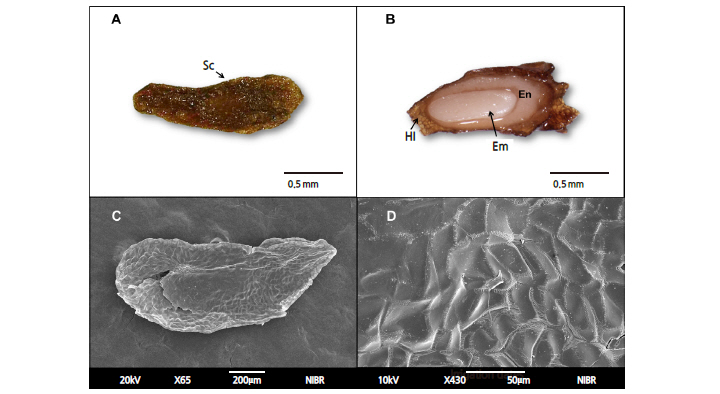
Well-selected seeds were cut along the major or minor axis using a stainless blade (Platinum ST-300, Dorco, Wonju, Korea). The cross-sections were photographed with an iCamScope (ICS, Sometech Inc., Seoul, Korea) and observed using IT Plus 4.0 software. The ratio of embryo length to seed length was calculated (E:S ratio, %) (Vandelook et al., 2007). Embryo development status was distinguished by criteria such as whether the cotyledon was differentiated or developed but not differentiated and whether the embryo was immature or non-existent.
Well-selected seeds were submerged in distilled water for 24 hours and placed in Petri dishes 8.9 mm in diameter containing two sheets of filter paper (90 mm, Advantec, Toyo, Japan) using the top of paper method. The Petri dishes were placed in growth chambers under light (continuous light of 23 ± 0.5 μmol·m-2·s-1) or dark conditions at a temperature of 20°C. The sheets of filter paper were moistened with sterilized water every day to avoid drying, and the germination rate (%) was calculated by totaling all seeds germinated in 30 days.
The seeds were incubated in water for 7 days, after which the ratio of moisture content to moisture absorption was calculated. The seeds were places in test tubes containing 15 mL of distilled water and stored at 4 ± 1.0°C in a low-temperature warehouse. Every 24 hours, the seeds were removed. The seed coats were wiped with filter paper, weighed, and placed in fresh distilled water. The interval moisture absorption rate was calculated based on the cumulative moisture content in addition to the average weight of the seeds, which was calculated every 24 hours.
Seed Germination Conditions
The seeds were submerged in distilled water for 24 hours and placed in Petri dishes lined with two sheets of filter paper. The Petri dishes were then placed under various temperature and light conditions: in growth chambers at 15, 20, 25 and 30°C; light conditions, with a photosynthetic photon flux density of 23 ± 0.5 μmol·m-2·s-1 (LI-1400, LI-COR, Lincoln, NE, USA) provided by fluorescent lights (continuous light) or dark conditions.
Chemical treatments
To accelerate germination and to encourage uniform seedling growth, the seeds were treated with different concentrations of growth regulators and minerals. The seeds were submerged in 100, 200, or 500 mg·L-1 of GA3 (≥ 90%, Acros, NJ, USA) and 10 or 20 mg·L-1 of kinetin (99.0%, Alfa Aesar, Heysham, England) as plant growth regulators, and 5, 10, 20, or 40 mM potassium nitrate (KNO3, 99.0%, Junsei Chemical Co., Ltd., Tokyo, Japan) and 100, 200, or 500 mM potassium chloride (KCl, 99.0%, OCI Co. Ltd., Incheon, Korea) as minerals. The seeds were submerged in each solution at 4 ± 1.0°C for 24 hours and washed three times with distilled water. The germination characteristics of the seeds at 30°C under light conditions were analyzed.
Investigations and Statistical Analysis
Sterilized, distilled water was supplied every day to keep the filter paper moist, and any contaminated filter paper was immediately replaced. Germination was investigated every 24 hours, where a seed was defined as germinated if the radicle had broken through the seed coat by at least 1 mm.
Percent germination (PG, %) was calculated as the number of germinated seeds by the end of the experiment. Germination energy (GE, %) was calculated as the ratio of seeds germinated within 7 and 14 days of sowing to the total number of germinated seeds submerged in plant growth regulators and minerals, respectively. T50 indicates the number of days until 50% of the seeds had germinated.
Seed length, width, and 1,000-seed weight were measured 10 times, and all germination tests were repeated four times with 50 seeds. Moisture content and the interval moisture absorption rate were measured in four replicates with 100 seeds per replicate. Duncan’s multiple range test in SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) was used to establish statistical significance at the p < 0.05 level.
Results
Classification of Seed Dormancy Types on Well-selected Seeds
The seed size of A. tabularis averaged 2.07 × 0.66 mm and the 1,000-seed weight was 75.3 ± 0.14 mg. The seeds contained a distinguishable embryo and endosperm but lacked a cotyledon and hypocotyl (Figs. 1A and 1B). No organ for moisture absorption was observed on the seed coat (Figs. 1C and 1D). The E:S ratio of the seeds was 34.6%, and direct sowing experiments of undifferentiated seeds led to germination within 4 weeks without fail. The germination rate, however, was only 45.9% (data not shown).
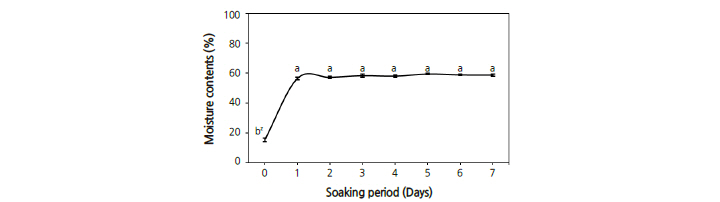
The moisture content of the seeds was 15.4%, which reached a maximum of 56.4% within 24 hours and maintained a similar level for 7 days (Fig. 2).
Seed Germination Conditions
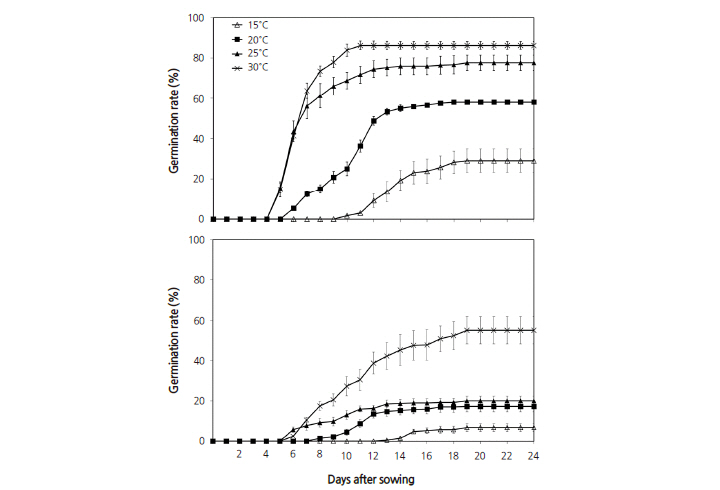
We examined the germination rates of A. tabularis seeds in germination beds at different temperatures and light conditions. The germination rate was highest (86.3%) at 30°C under light conditions; this rate was generally higher in the light than in the dark (Fig. 3). Regardless of light conditions, the germination rates were higher at higher temperatures. The days to germination were shorter at all temperatures under light conditions; germination was complete within 11 days at 30°C in the light. At the same temperature under dark conditions, germination continued to day 19, as dark conditions generally hampered germination.
| |
Fig. 3. Effects of light and temperature on seed germination in Astilboides tabularis (Hemsl.) Engl. Bars represent standard errors (n = 4) | |
Effects of Chemical Treatments on Seed Germination
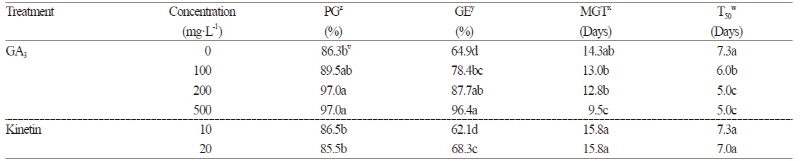
Seeds of A. tabularis were treated with various concentrations of GA3 to improve the germination rate. Treatment with both 200 and 500 mg·L-1 of GA3 led to an excellent germination rate of 97.0%, and 100 mg·L-1 of GA3 improved the germination rate compared to the control (Table 1). GA3 treatment improved the GE of A. tabularis seeds significantly, with 500 mg·L-1 being the most effective treatment (31.5%; Table 1). Furthermore, with higher GA3 concentrations, shorter mean germination time (MGT) and T50 were observed. At 500 mg·L-1 of GA3, the MGT and T50 were reduced by 4.8 and 2.3 days, respectively.
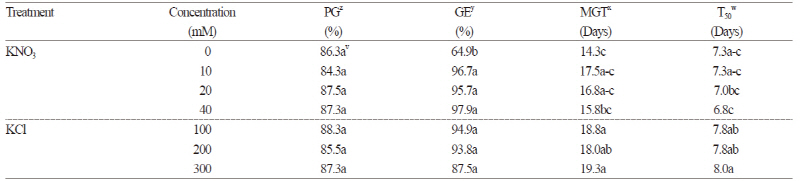
Kinetin treatment did not improve the germination rate, and GE was reduced by 20 mg·L-1 kinetin treatment (Table 1). Treatment with varying concentrations of KNO3 and KCl also failed to improve the germination rate (Table 2). However, MGT and T50 were significantly reduced with increasing KNO3 concentration, and GE was improved by at least 30% at all concentrations.
Discussion
If freshly matured seeds show a germinate rate of over 80% within 4 weeks, they are classified as non-dormant seeds (ND). By contrast, seeds that have a germination rate below 80% within 4 weeks of exposure to germination conditions and an undifferentiated or underdeveloped embryo can be classified as morphologically dormant (MD) (Baskin and Baskin, 2004b). Therefore, the direct sowing germination rate and embryo development are important factors for distinguishing ND from MD.
The levels of MD, as distinguished by the degree of development and differentiation, can be similar in different families. Seeds of Burmanniaceae, Ochidaceae, Orobanchaceae, and Rafflesiaceae have an undifferentiated embryo and are dispersed without the formation of a radicle and cotyledon. Certain Apiaceae and Ranunculaceae seeds have a differentiated but underdeveloped embryos. These seeds also possess a radicle and cotyledon, although they are less than 1 mm in length (Baskin and Baskin, 1998).
In this study, seeds of A. tabularis were confirmed to have a developed and undifferentiated embryo (Fig. 1) and low germination rate below 50%. Seven days of soaking allowed the seeds to reach the maximum moisture content (Fig. 2). These findings suggest that the seed coat is not impermeable and the seeds are not in physical dormancy (Baskin and Baskin, 1998). Thus, the seeds of A. tabularis were classified as MD.
Each plant has an optimum germination temperature, which is highly related to the environment of the native habitat (Bewley and Black, 1982). Seeds native to temperate regions have an optimum germination temperature between 24 and 30°C (Hartmann et al., 1997). Seeds of A. tabularis were classified as MD in this study, and the best germination rate was obtained at 30°C under light conditions, confirming the characteristics of plants native to temperate regions. The optimum germination temperature of native Orostachys japonicus, O. iwarenge (Kang et al., 2010) is 10°C. Also, maximum germination rates for Hydrangea petiolaris (Cho et al., 2014) is obtained at 25°C and in the light, and germination is severely restrained in the dark. Therefore, optimum germination temperatures are thought to vary by species but share similarities intergenerically.
Growth regulators interact with plants at concentrations lower than 10-6 M (Bewley, 1997). The growth regulator kinetin is a type of cytokinin that affects plant cell division, as well as the formation of aboveground plant parts in tissue culture, anti-aging of plants, seed germination, and other processes (Miller, 1956; Wittwer and Dedolph, 1963). GA is a well-known growth regulator that induces physiological reactions such as dormancy breaking, germination acceleration, flower bud formation, flowering acceleration, and the activation of hydrolytic enzymes (Blázquez et al., 1998; Groot and Karssen, 1987; Gubler et al., 1995; Pharis and King, 1985; Wilson et al., 1992). GA accelerates germination in barley and rice, as GA biosynthesized in the embryo interacts with the seed’s aleurone layer and catalyzes α-amylase secretion. Activated α-amylase decomposes starch and proteins in the endosperm to accelerate embryo development, which leads to accelerated germination (Abeles, 1986; Kim et al., 2009).
In the current study, we found that GA3 improved the germination rate and shortened the days to seed germination in A. tabularis, but relatively low concentrations of kinetin were ineffective (Table 1). KNO3 treatment was effective at reducing the period of germination. Germination of Crotalaria sessiliflora seeds can be accelerated by 12-hour-long treatment with 0.1 mM of GA3 (Kang et al., 2001), and in the native plant Primula sachalinensis, exogenous GA treatment can break seed dormancy and initiate germination (Song and Lee, 2002).
Seed priming, including hydropriming, osmopriming, and matripriming with inorganic salts, is a seed hydration technique used to control seed absorption, thereby improving the germination rate. In addition, uniform germination can be achieved by treatment with inorganic salts under a broad temperature range (Bittencourt et al., 2004; Fay et al., 1994; Smith and Cobb, 1991). KNO3 used in priming treatment accelerates germination of Lysimachia vulgaris var. davurica seeds at higher concentrations (Lee et al., 2003) and is also effective at accelerating the germination of Exochorda serratifolia seeds (Lee et al., 2006), Adenophora triphylla var. japonica seeds (Kim et al., 1996), and others. However, the germination of Caltha palustris L. var. palustris seeds (La and Jeong, 2008) is not accelerated by treatment with inorganic salts. In Allium cepa L.seeds, KCl priming increases the germination speed, but other inorganic substances including KNO3, NH4NO3, and K3PO4 result in low germination rates compared to non-treated seeds (Cho et al., 2006). Therefore, the germination rate is accelerated by priming treatment, although the sensitivity varies according to chemical type, concentration, soaking duration and temperature, as well as plant species.
Consequently, the effects of GA3 and KNO3 on germination acceleration were confirmed in the seeds of A. tabularis, while kinetin and KCl had little effect. These results help confirm the notion that the effects of growth regulators and minerals on germination vary by species and concentration. Furthermore, our results indicate that GA3 treatment is effective for consistent seedling growth and the preservation of A. tabularis.