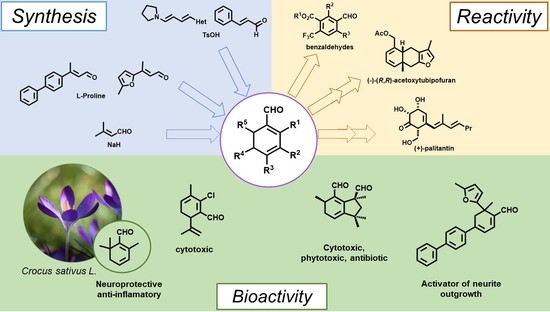
1,3-Cyclohexadien-1-Als: Synthesis, Reactivity and Bioactivities
Abstract
:1. Introduction
2. Synthesis of 1,3-Cyclohexadien-1-Al Scaffold
2.1. Functional Group Interconversions and Isomerizations
2.2. Oxidation
2.3. Vilsmeier–Haack Formylation
2.4. Aldol-Type Reactions and Horner Olefinations
2.5. Pericyclic Reactions: Diels–Alder and Electrocyclizations
2.6. Organometallic Synthetic Procedures
2.7. Organocatalytic Methodologies
3. Reactivity of 1,3-Cyclohexadien-1-Als
3.1. Reactions Involving Double Bond(s)
3.2. Reactions Involving the Aldehyde Functional Group
3.3. Reactions Involving the α,β-Unsaturated Aldehyde System
4. Biological Activities of 1,3-Cyclohexadien-1-Carboxaldehyde
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Diels, O.; Alder, K. Syntheses in the hydroaromatic series. I. Addition of “diene” hydrocarbons. Justus Liebigs Ann. Chem. 1928, 460, 98–122. [Google Scholar] [CrossRef]
- Griesser, M.; Weingart, G.; Schoedl-Hummel, K.; Neumann, N.; Becker, M.; Varmuza, K.; Liebner, F.; Schuhmacher, R.; Forneck, A. Severe drought stress is affecting selected primary metabolites, polyphenols, and volatile metabolites in grapevine leaves (Vitis vinifera cv. Pinot noir). Plant Physiol. Biochem. 2015, 88, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Mastelić, J.; Jerković, I.; Mesić, M. Volatile constituents from flowers, leaves, bark and wood of Prunus mahaleb L. Flavour Fragr. J. 2006, 21, 306–313. [Google Scholar] [CrossRef]
- Tarantilis, P.A.; Tsoupras, G.; Polissiou, M. Determination of saffron (Crocus sativus L.) components in crude plant extract using high-performance liquid chromatography-UV-visible photodiode-array detection-mass spectrometry. J. Chromatogr. A 1995, 699, 107–118. [Google Scholar] [CrossRef]
- Peters, L.; Wright, A.D.; Krick, A.; König, G.M. Variation of brominated indoles and terpenoids within single and different colonies of the marine bryozoan Flustra foliacea. J. Chem. Ecol. 2004, 30, 1165–1181. [Google Scholar] [CrossRef] [PubMed]
- Starkenmann, C.; Mayenzet, F.; Brauchli, R.; Wunsche, L.; Vial, C. Structure Elucidation of a Pungent Compound in Black Cardamom: Amomum Tsao-Ko Crevost Et Lemarié (Zingiberaceae). J. Agric. Food Chem. 2007, 55, 10902–10907. [Google Scholar]
- Reino, J.L.; Durán-Patrón, R.; Segura, I.; Hernández-Galán, R.; Riese, H.H.; Collado, I.G. Chemical transformations on botryane skeleton. Effect on the cytotoxic activity. J. Nat. Prod. 2003, 66, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Thippeswamy, B.; Sushma, N.; Naveenkumar, K. Evaluation of antimicrobial property of lichen-Parmelia perlata. Afr. J. Pharm. Pharmacol. 2013, 7, 1242–1250. [Google Scholar]
- Jacques, B.; Eloi, A.; Chavarot-Kerlidou, M.; Rose-Munch, F.; Rose, E.; Gérard, H.; Herson, P. Unprecedented (η5-Formylcyclohexadienyl)Mn(CO)3 Complexes: Synthesis, Structural and Theoretical Characterizations, and Resolution of the Planar Chirality. Organometallics 2008, 27, 2505–2517. [Google Scholar]
- Papandreou, M.A.; Kanakis, C.D.; Polissiou, M.G.; Efthimiopoulos, S.; Cordopatis, P.; Margarity, M.; Lamari, F.N. Inhibitory activity on amyloid-β aggregation and antioxidant properties of Crocus sativus stigmas extract and its crocin constituents. J. Agric. Food Chem. 2006, 54, 8762–8768. [Google Scholar] [CrossRef]
- Lambrianidou, A.; Koutsougianni, F.; Papapostolou, I.; Dimas, K. Recent Advances on the Anticancer Properties of Saffron (Crocus sativus L.) and Its Major Constituents. Molecules 2021, 26, 86. [Google Scholar] [CrossRef] [PubMed]
- Boulin, B.; Arreguy-San Miguel, B.; Delmond, B. Le γ-Pyronène: Synthon d’Accès au Safranal et Précurseur d’Intermédiaires de Synthèse de la Forskoline et du Strigol. Tetrahedron 1998, 54, 2753–2762. [Google Scholar] [CrossRef]
- van Wijk, A.A.C.; van de Weerd, M.B.; Lugtenburg, J. Synthetic Scheme for the Preparation of 13C-Labeled 3,4-Didehydro-Retinal, 3-Hydroxyretinal, and 4-Hydroxyretinal up to Uniform 13C-Enrichment. Eur. J. Org. Chem. 2003, 2003, 863–868. [Google Scholar] [CrossRef]
- Durán-Patrón, R.; Hernández-Galán, R.; Rebordinos, L.G.; Cantoral, J.M.; Collado, I.G. Structure-activity relationships of new phytotoxic metabolites with the botryane skeleton from Botrytis cinerea. Tetrahedron 1999, 55, 2389–2400. [Google Scholar] [CrossRef]
- Emmanuel Hatzakis, I.O.; Bogdan, A. Solaja and Manolis Stratakis Synthesis of Novel Polar Derivatives of the Antimalarial Endoperoxides Ascaridole and Dihydroascaridole. ARKIVOC 2007, 8, 124–135. [Google Scholar]
- Pacheco, J.J.; Davis, M.E. Synthesis of terephthalic acid via Diels-Alder reactions with ethylene and oxidized variants of 5-hydroxymethylfurfural. Proc. Natl. Acad. Sci. USA 2014, 111, 8363–8367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacheco, J.J.; Labinger, J.A.; Sessions, A.L.; Davis, M.E. Route to Renewable PET: Reaction Pathways and Energetics of Diels–Alder and Dehydrative Aromatization Reactions Between Ethylene and Biomass-Derived Furans Catalyzed by Lewis Acid Molecular Sieves. ACS Catal. 2015, 5, 5904–5913. [Google Scholar] [CrossRef]
- Kutney, J.P.; Gunning, P.J.; Clewley, R.G.; Somerville, J.; Rettig, S.J. The chemistry of thujone. XVI. Versatile and efficient routes to safronitrile, β-cyclogeranonitrile, β-cyclocitral, damascones, and their analogues. Can. J. Chem. 1992, 70, 2094–2114. [Google Scholar] [CrossRef] [Green Version]
- Gogoi, J.; Bezbaruah, P.; Saikia, P.; Goswami, J.; Gogoi, P.; Boruah, R.C. Synthesis of a novel class of steroidal tetrazolo[1,5-a]pyridines via intramolecular 1,3-dipolar cycloadditions. Tetrahedron Lett. 2012, 53, 1497–1500. [Google Scholar] [CrossRef]
- Jones, G.; Stanforth, S.P. The Vilsmeier Reaction of Non-Aromatic Compounds. In Organic Reactions; Overman, L.E., Ed.; Willey: Hoboken, NJ, USA, 2004; Volume 56, pp. 355–686. [Google Scholar]
- Laurent, H.; Wiechert, R. Synthesis and reactions of 3-chlor-2-formyl-steroids. Chem. Ber. 1968, 101, 2393–2403. [Google Scholar] [CrossRef]
- Consomi, A.; Mancini, F.; Pallini, U.; Patelli, B.; Sciaki, R. Reactions of dienamines and dienol ethers. Gazz. Chim. Ital. 1970, 100, 244. [Google Scholar]
- Laurent, H.; Schulz, G.; Wiechert, R. Notiz über die Darstellung von 7-Chlor-2-formyl-Δ2.4.6-steroiden. Chem. Ber. 1966, 99, 3057–3059. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Marson, C.M.; Wang, Z. Reactions of alkyl-substituted 2-cyclohexen-1-ones with Vilsmeier reagents. J. Org. Chem. 1987, 52, 2730–2734. [Google Scholar] [CrossRef]
- Escher, I.; Glorius, F. Science of Synthesis; Thieme Chemistry; Georg Thieme Verlag KG Stuttgart: Stuttgart, Germany, 2007; Volume 25, p. 711. [Google Scholar]
- Lauro, F.-V.; Francisco, D.C.; Marcela, R.-N.; Virginia, M.-A.; Elodia, G.-C.; Eduardo, P.G.; Lenin, H.; Maria, L.-R.; Alondra, L.-J.; Jhair, C.-T. Design and Synthesis of two Steroid-diazocine derivatives. Heterocycl. Lett. 2018, 8, 745–753. [Google Scholar]
- Migneault, I.; Dartiguenave, C.; Bertrand, M.J.; Waldron, K.C. Glutaraldehyde: Behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. BioTechniques 2004, 37, 798–802. [Google Scholar] [CrossRef]
- Zhang, H.; Fei, J.; Yan, X.; Wang, A.; Li, J. Enzyme-Responsive Release of Doxorubicin from Monodisperse Dipeptide-Based Nanocarriers for Highly Efficient Cancer Treatment In Vitro. Adv. Funct. Mater. 2015, 25, 1193–1204. [Google Scholar] [CrossRef]
- Verdegem, P.J.E.; Monnee, M.C.F.; Mulder, P.P.J.; Lugtenburg, J. Condensation of all-E-retinal. Tetrahedron Lett. 1997, 38, 5355–5358. [Google Scholar] [CrossRef]
- Dyakonova, I.A.; Cherkaev, G.V.; Erman, M.B. Alkali-catalysed Self-condensation of 3-Methylbut-2-enal: Formation of Novel Rosoxide Dehydro Analogue. Mendeleev Commun. 1994, 4, 88. [Google Scholar] [CrossRef]
- Kurokawa, H.; Yanai, M.; Ohshima, M.-A.; Miura, H. Cyclodimerization of crotonaldehyde to form cyclohexadienecarbaldehydes and tolaldehydes over solid base catalysts. React. Kinet. Mech. Catal. 2012, 105, 401–412. [Google Scholar] [CrossRef]
- Duhamel, L.; Guillemont, J.; Poirier, J.-M.; Chabardes, P. A new prenylation method using the lithium enolate of prenal. Reaction with aldehydes and α,β-unsaturated aldehydes. Tetrahedron Lett. 1991, 32, 4495–4498. [Google Scholar] [CrossRef]
- Valla, A.; Andriamialisoa, Z.; Labia, R. Synthesis of new cyclic retinoids via base induced self-condensation of C-13, C-14 and C-15 units. Tetrahedron Lett. 2000, 41, 3359–3362. [Google Scholar] [CrossRef]
- Moorhoff, C.M.; Schneider, D.F. Comments on the reaction of ethyl 4-(diethoxyphosphinyl)-3-oxobutanoate and related phosphonate esters with enals. Tetrahedron Lett. 1987, 28, 559–562. [Google Scholar] [CrossRef]
- Moorhoff, C.M.; Schneider, D.F. Condensation of ethyl and methyl 4-(triphenylphosphoranylidine)-3-oxobutanoate with enals. Tetrahedron Lett. 1987, 28, 4721–4724. [Google Scholar] [CrossRef]
- Ye, L.-W.; Han, X.; Sun, X.-L.; Tang, Y. Tandem Michael addition/ylide olefination reaction for the synthesis of highly functionalized cyclohexadiene derivatives. Tetrahedron 2008, 64, 8149–8154. [Google Scholar] [CrossRef]
- Marshall, J.A.; Audia, J.E.; Shearer, B.G. Conjugate addition of organocuprates to trans-4a,5,6,7,8,8a-hexahydronaphthalene-1-carboxaldehydes. Synthesis of a chlorothricin degradation product. J. Org. Chem. 1986, 51, 1730–1735. [Google Scholar] [CrossRef]
- De Groot, A.; Jansen, B.J.M. Diels-Alder Reaction of Exocyclic Sulfur-Substituted 1,3-Dienes. Synthesis 1978, 1978, 52–53. [Google Scholar] [CrossRef]
- Kenji, U.; Kunikazu, T.; Sigeru, T. 2-(4-Chlorophenylseleno)-2-butenal as a New Dienophile for Diels-Alder Reaction. Bull. Chem. Soc. Jpn. 1983, 56, 2867–2868. [Google Scholar] [CrossRef]
- Skowroriska, A.; Dybowski, P.; Koprowski, M.; Krawczyk, E. The Diels-Alder Reactions of (Z)-1,2-Diheterosubstituted-1,3-Dienes. Sulfur as a Regiochemical Control. Tetrahedron Lett. 1995, 36, 8133–8136. [Google Scholar] [CrossRef]
- Koprowski, M.; Skowrońska, A.; Główka, M.L.; Fruziński, A. Fully regio- and endo-stereoselective synthesis of new polycyclic allylic sulfides via a Diels–Alder reaction. Synthetically useful transformations of these sulfides. Tetrahedron 2007, 63, 1211–1228. [Google Scholar] [CrossRef]
- Kotschy, A.; Hajós, G.; Timári, G.; Messmer, A.; Schantl, J.G. “Ionic Diels-Alder Reaction” of Hetaryldienamines. Heterocycl. Commun. 1997, 3, 449. [Google Scholar] [CrossRef]
- Brandt, K.; Haas, A.; Hardt, T.; Mayer-Figge, H.; Merz, K.; Wallmichrath, T. Synthesis of geminal-bis(trifluoromethyl)-substituted dienes, heterodienes, 7,7,7,8,8,8-hexafluoro-β-cyclocitral and -safranal. J. Fluor. Chem. 1999, 97, 115–125. [Google Scholar] [CrossRef]
- Zhou, S.; Sánchez-Larios, E.; Gravel, M. Scalable Synthesis of Highly Reactive 1,3-Diamino Dienes from Vinamidinium Salts and Their Use in Diels–Alder Reactions. J. Org. Chem. 2012, 77, 3576–3582. [Google Scholar] [CrossRef]
- Bishop, L.M.; Roberson, R.E.; Bergman, R.G.; Trauner, D. On the Development of Catalytic Carba-6π Electrocyclizations. Synthesis 2010, 2010, 2233–2244. [Google Scholar] [CrossRef] [Green Version]
- Varela, J.A.; Rubín, S.G.; González-Rodríguez, C.; Castedo, L.; Saá, C. A New Ru-Catalyzed Cascade Reaction Forming Polycyclic Cyclohexadienes from 1,6-Diynes and Alkenes. J. Am. Chem. Soc. 2006, 128, 9262–9263. [Google Scholar] [CrossRef]
- Corey, E.J.; Kozikowski, A.P. Bis(dimethylaluminum) 1,3-propanedithiolate–a useful reagent in the conversion of esters to unsaturated aldehydes and ketones. Tetrahedron Lett. 1975, 16, 925–928. [Google Scholar] [CrossRef]
- Kuendig, E.P.; Ripa, A.; Liu, R.; Bernardinelli, G. Arenes to Substituted Cyclohexadienes: Nucleophile/Electrophile Additions across an Arene Double Bond. J. Org. Chem. 1994, 59, 4773–4783. [Google Scholar] [CrossRef]
- Amurrio, D.; Khan, K.; Kündig, E.P. Asymmetric Addition of Organolithium Reagents to Prochiral Arene Tricarbonylchromium Complexes. J. Org. Chem. 1996, 61, 2258–2259. [Google Scholar] [CrossRef]
- Bernardinelli, G.; Gillet, S.; Kündig, E.P.; Liu, R.; Ripa, A.; Saudan, L. Diastereoselective Transformation of Arenes into Highly Enantiomerically Enriched Substituted Cyclohexadienes. Synthesis 2001, 2001, 2040–2054. [Google Scholar] [CrossRef]
- Kündig, E.P.; Cannas, R.; Laxmisha, M.; Ronggang, L.; Tchertchian, S. Chromium-Mediated Asymmetric Synthesis of Both Enantiomers of Acetoxytubipofuran. J. Am. Chem. Soc. 2003, 125, 5642–5643. [Google Scholar] [CrossRef] [PubMed]
- Kündig, E.P.; Laxmisha, M.S.; Cannas, R.; Tchertchian, S.; Ronggang, L. Chromium-Mediated Dearomatization: Application to the Synthesis of Racemic 15-Acetoxytubipofuran and Asymmetric Synthesis of Both Enantiomers. Helv. Chim. Acta 2005, 88, 1063–1080. [Google Scholar] [CrossRef]
- Bench, B.J.; Liu, C.; Evett, C.R.; Watanabe, C.M.H. Proline Promoted Synthesis of Ring-Fused Homodimers: Self-Condensation of α,β-Unsaturated Aldehydes. J. Org. Chem. 2006, 71, 9458–9463. [Google Scholar] [CrossRef]
- Yamada, S.-I.; Shibasaki, M.; Terashima, S. A biogenetic-type asymmetric cyclization syntheses of optically active α-cyclocitral and trans-α-damascone. Tetrahedron Lett. 1973, 14, 381–384. [Google Scholar] [CrossRef]
- Yamada, S.-I.; Shibasaki, M.; Terashima, S. A new biogenetic-type cyclization of citral to α-cyclocitral via an enamine. Tetrahedron Lett. 1973, 14, 377–380. [Google Scholar] [CrossRef]
- Asato, A.E.; Watanabe, C.; Li, X.-Y.; Liu, R.S.H. The proline and β-lactoglobulin mediated asymmetric self-condensation of β-ionylideneacetaldehyde, retinal and related compounds. Tetrahedron Lett. 1992, 33, 3105–3108. [Google Scholar] [CrossRef]
- Paduraru, M.P.; Wilson, P.D. Synthesis of the Polycyclic Ring Systems of Artocarpol A and D. Org. Lett. 2003, 5, 4911–4913. [Google Scholar] [CrossRef] [PubMed]
- Peña, J.; Moro, R.F.; Basabe, P.; Marcos, I.S.; Díez, D. Solvent free l-proline-catalysed domino Knoevenagel/6π-electrocyclization for the synthesis of highly functionalised 2H-pyrans. RSC Adv. 2012, 2, 8041–8049. [Google Scholar] [CrossRef]
- Weber, A.K.; Schachtner, J.; Fichtler, R.; Leermann, T.M.; Neudörfl, J.M.; Jacobi von Wangelin, A. Modular synthesis of cyclic cis- and trans-1,2-diamine derivatives. Org. Biomol. Chem. 2014, 12, 5267–5277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, Y.; Kawamoto, Y.; Honda, M.; Okamura, D.; Umemiya, S.; Noguchi, Y.; Mukaiyama, T.; Sato, I. Diphenylprolinol Silyl Ether Catalyzed Asymmetric Michael Reaction of Nitroalkanes and β,β-Disubstituted α,β-Unsaturated Aldehydes for the Construction of All-Carbon Quaternary Stereogenic Centers. Chem. A Eur. J. 2014, 20, 12072–12082. [Google Scholar] [CrossRef]
- Hong, B.-C.; Wu, M.-F.; Tseng, H.-C.; Liao, J.-H. Enantioselective Organocatalytic Formal [3 + 3]-Cycloaddition of α,β-Unsaturated Aldehydes and Application to the Asymmetric Synthesis of (−)-Isopulegol Hydrate and (−)-Cubebaol. Org. Lett. 2006, 8, 2217–2220. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.-C.; Tseng, H.-C.; Chen, S.-H. Synthesis of aromatic aldehydes by organocatalytic [4+2] and [3+3] cycloaddition of α,β-unsaturated aldehydes. Tetrahedron 2007, 63, 2840–2850. [Google Scholar] [CrossRef]
- Bench, B.J.; Tichy, S.E.; Perez, L.M.; Benson, J.; Watanabe, C.M.H. Synthesis and cellular effects of cycloterpenals: Cyclohexadienal-based activators of neurite outgrowth. Bioorganic Med. Chem. 2008, 16, 7573–7581. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Wong, W.-T.; Yang, D. Organocatalyzed Asymmetric Synthesis of Dihydrodibenzofurans Based on a Dienamine Process. Org. Lett. 2013, 15, 4980–4983. [Google Scholar] [CrossRef]
- Brindani, N.; Rassu, G.; Dell’Amico, L.; Zambrano, V.; Pinna, L.; Curti, C.; Sartori, A.; Battistini, L.; Casiraghi, G.; Pelosi, G.; et al. Organocatalytic, Asymmetric Eliminative [4+2] Cycloaddition of Allylidene Malononitriles with Enals: Rapid Entry to Cyclohexadiene-Embedding Linear and Angular Polycycles. Angew. Chem. Int. Ed. 2015, 54, 7386–7390. [Google Scholar] [CrossRef]
- Urosa, A.; Tobal, I.E.; de la Granja, Á.P.; Capitán, M.C.; Moro, R.; Marcos, I.S.; Garrido, N.M.; Sanz, F.; Calle, E.; Díez, D. Diastereoselective synthesis of chiral 1,3-cyclohexadienals. PLoS ONE 2018, 13, e0192113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saktura, M.; Frankowski, S.; Joachim, B.; Albrecht, Ł. Aminocatalytic Synthesis of Uracil Derivatives Bearing a Bicyclo[2.2.2]octane Scaffold via a Doubly Cycloadditive Reaction Cascade. Synthesis 2020. [Google Scholar] [CrossRef]
- Hammer, N.; Leth, L.A.; Stiller, J.; Jensen, M.E.; Jørgensen, K.A. Oxadendralenes in asymmetric organocatalysis for the construction of tetrahydroisochromenes. Chem. Sci. 2016, 7, 3649–3657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vosburg, D.A.; Weiler, S.; Sorensen, E.J. A Concise Synthesis of Fumagillol. Angew. Chem. Int. Ed. 1999, 38, 971–974. [Google Scholar] [CrossRef]
- Vosburg, D.A.; Weiler, S.; Sorensen, E.J. Concise stereocontrolled routes to fumagillol, fumagillin, and TNP-470. Chirality 2003, 15, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.-C.; Wu, M.-F.; Tseng, H.-C.; Huang, G.-F.; Su, C.-F.; Liao, J.-H. Organocatalytic Asymmetric Robinson Annulation of α,β-Unsaturated Aldehydes: Applications to the Total Synthesis of (+)-Palitantin. J. Org. Chem. 2008, 73, 2480. [Google Scholar] [CrossRef] [Green Version]
- Yamaji, K.; Fukushi, Y.; Hashidoko, Y.; Yoshida, T.; Tahara, S. Characterization of Antifungal Metabolites Produced by Penicillium Species Isolated from Seeds of Picea glehnii. J. Chem. Ecol. 1999, 25, 1643–1653. [Google Scholar] [CrossRef]
- Curtis, P.J.; Hemming, H.G.; Smith, W.K. Frequentin; an antibiotic produced by some strains of Penicillium frequentans Westling. Nature 1951, 167, 557–558. [Google Scholar] [CrossRef]
- Trost, B.M.; Huang, Z.; Murhade, G.M. Catalytic palladium-oxyallyl cycloaddition. Science 2018, 362, 564. [Google Scholar] [CrossRef] [Green Version]
- Jablonski, C.R.; Sorensen, T.S. Acid Catalyzed Rearrangements of Structurally Constrained Tricarbonyl(trans-pentadienyl)iron Cations. Can. J. Chem. 1974, 52, 2085–2097. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.; Belley, M. New methods of formation of meta-substituted aromatic compounds. J. Org. Chem. 1986, 51, 3878–3881. [Google Scholar] [CrossRef]
- Peng, F.; Zhao, Q.; Huang, W.; Liu, S.-J.; Zhong, Y.-J.; Mao, Q.; Zhang, N.; He, G.; Han, B. Amine-catalyzed and functional group-controlled chemo- and regioselective synthesis of multi-functionalized CF3-benzene via a metal-free process. Green Chem. 2019, 21, 6179–6186. [Google Scholar] [CrossRef]
- Hennig, A.; Schwarzlose, T.; Nau, W.M. Bridgehead carboxy-substituted 2,3-diazabicyclo[2.2.2]oct-2-enes: Synthesis, fluorescent properties, and host-guest complexation. Arkivoc 2007, 8, 341–357. [Google Scholar] [CrossRef] [Green Version]
- Griesbeck, A.G.; de Kiff, A.; Neudörfl, J.M.; Sillner, S. Singlet oxygen addition to cyclo-1,3-hexadienes from natural sources and from organocatalytic enal dimerization. Arkivoc 2015, 3, 101–110. [Google Scholar] [CrossRef]
- Lagnel, B.M.F.; Morin, C.; De Groot, A. Synthesis of drimanes from (+)-larixol. Synthesis 2000, 13, 1907–1916. [Google Scholar] [CrossRef]
- Razmilic, I.; López, J.; Sierra, J. An Alternative Partial Synthesis of (-)-Polygodial. Synth. Commun. 1987, 17, 95–103. [Google Scholar] [CrossRef]
- Oyarzún, M.L.; Cortés, M.; Sierra, J. Synthesis of (-)-DRIM-7-ENE-9α, 11, 12 Triol. The Direct Precursor of (-)-Warburganal. Synth. Commun. 1982, 12, 951–958. [Google Scholar] [CrossRef]
- Denmark, S.E.; Hite, G.A. Silicon-Directed Nazarov Cyclizations. Part VI. The anomalous cyclization of vinyl dienyl ketones. Helv. Chim. Acta 1988, 71, 195–208. [Google Scholar] [CrossRef]
- Bennani, Y.L.; Boehm, M.F. Syntheses of High Specific Activity 2,3- and 3,4-[3H]2-9-cis-Retinoic Acid. J. Org. Chem. 1995, 60, 1195–1200. [Google Scholar] [CrossRef]
- Frichert, A.; Jones, P.G.; Lindel, T. Synthesis of eunicellane-type bicycles embedding a 1,3-cyclohexadiene moiety. Beilstein J. Org. Chem. 2018, 14, 2461–2467. [Google Scholar] [CrossRef]
- Semmelhack, M.F.; Sarpong, R.; Bergman, J.; Ho, D.M. Evaluation of alkene isomerization as a trigger for enediyne activation. Tetrahedron Lett. 2002, 43, 541–544. [Google Scholar] [CrossRef]
- Dogra, A.; Kotwal, P.; Gour, A.; Bhatt, S.; Singh, G.; Mukherjee, D.; Nandi, U. Description of Druglike Properties of Safranal and Its Chemistry behind Low Oral Exposure. ACS Omega 2020, 5, 9885–9891. [Google Scholar] [CrossRef] [Green Version]
- Rezaee, R.; Hosseinzadeh, H. Safranal: From an aromatic natural product to a rewarding pharmacological agent. Iran. J. Basic Med. Sci. 2013, 16, 12. [Google Scholar] [PubMed]
- Melnyk, J.P.; Wang, S.; Marcone, M.F. Chemical and biological properties of the world’s most expensive spice: Saffron. Food Res. Int. 2010, 43, 1981–1989. [Google Scholar] [CrossRef]
- Sadeghnia, H.R.; Shaterzadeh, H.; Forouzanfar, F.; Hosseinzadeh, H. Neuroprotective effect of safranal, an active ingredient of Crocus sativus, in a rat model of transient cerebral ischemia. Folia Neuropathol. 2017, 55, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ma, J.; Fan, L.; Zou, Y.; Dang, X.; Wang, K.; Song, J. Neuroprotective effects of safranal in a rat model of traumatic injury to the spinal cord by anti-apoptotic, anti-inflammatory and edema-attenuating. Tissue Cell 2015, 47, 291–300. [Google Scholar] [CrossRef]
- Ali, M.S.; Al-Lohedan, H.A. Experimental and computational investigation on the molecular interactions of safranal with bovine serum albumin: Binding and anti-amyloidogenic efficacy of ligand. J. Mol. Liq. 2019, 278, 385–393. [Google Scholar] [CrossRef]
- Bharti, S.; Golechha, M.; Kumari, S.; Siddiqui, K.M.; Arya, D.S. Akt/GSK-3β/eNOS phosphorylation arbitrates safranal-induced myocardial protection against ischemia–reperfusion injury in rats. Eur. J. Nutr. 2012, 51, 719–727. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Sadeghnia, H.R. Safranal, a constituent of Crocus sativus (saffron), attenuated cerebral ischemia induced oxidative damage in rat hippocampus. J. Pharm. Pharm. Sci. 2005, 8, 394–399. [Google Scholar] [PubMed]
- Sadeghnia, H.R.; Kamkar, M.; Assadpour, E.; Boroushaki, M.T.; Ghorbani, A. Protective effect of safranal, a constituent of Crocus sativus, on quinolinic acid-induced oxidative damage in rat hippocampus. Iran. J. Basic Med. Sci. 2013, 16, 73. [Google Scholar]
- Samarghandian, S.; Azimi-Nezhad, M.; Samini, F. Preventive effect of safranal against oxidative damage in aged male rat brain. Exp. Anim. 2015, 64, 65–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, K.-J.; Yang, J.-S. Anti-allodynia effect of safranal on neuropathic pain induced by spinal nerve transection in rat. Int. J. Clin. Exp. Med. 2014, 7, 4990. [Google Scholar] [PubMed]
- Fukui, H.; Toyoshima, K.; Komaki, R. Psychological and neuroendocrinological effects of odor of saffron (Crocus sativus). Phytomedicine 2011, 18, 726–730. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Younesi, H.M. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. Bmc Pharmacol. 2002, 2, 7. [Google Scholar]
- Colmenares, A.; Aleu, J.; Duran-Patron, R.; Collado, I.; Hernandez-Galan, R. The putative role of botrydial and related metabolites in the infection mechanism of Botrytis cinerea. J. Chem. Ecol. 2002, 28, 997–1005. [Google Scholar] [CrossRef]
- Anzaldi, M.; Sottofattori, E.; Dusatti, F.; Ferro, M.; Pani, M.; Balbi, A. Synthesis of ionones and carvone analogues: Olfactory properties and preliminary toxicity assays. Eur. J. Med. Chem. 2000, 35, 797–803. [Google Scholar] [CrossRef]
- Socha, A.M.; LaPlante, K.L.; Rowley, D.C. New bisanthraquinone antibiotics and semi-synthetic derivatives with potent activity against clinical Staphylococcus aureus and Enterococcus faecium isolates. Bioorganic Med. Chem. 2006, 14, 8446–8454. [Google Scholar] [CrossRef]

































































 | |||||
| Compound | R1 | R2 | R3 | R4 | % Yield (ee) |
| 50 | Me | H | H | Me | 82 (--) |
| 150 | H | αOAc | αCH2OAc | H | 68 (94) |
| 151 | H | H | αPh | H | 61 (63) |
| 152 | Me | H | αPh | H | 72 (32) |
| 153 | Me | H | αPh | H | 78 (56) |
| 154 | Me | H | αEt | H | 71 (--) |
| 155 | Me | H | αMe | H | 82 (41) |
 | |||
| Compound | Activity | Test | Reference |
| 2 | Anticancer, antioxidant, neuroprotective, anti-inflammatory | In vitro | [11,87,88,89,90,91,92,93,94,95,96,97,98,99] |
| 6 | Cytotoxic, phytotoxic, antibiotic | In vitro | [7,14,100] |
| 7 | Antibacterial, antifungal | In vitro | [8] |
| 36 | Cytotoxic | In vitro | [101] |
| 208 | Antimalaric | [15,79] | |
| 233 | Antibacterial | In vitro | [102] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tobal, I.E.; Bautista, R.; Diez, D.; Garrido, N.M.; García-García, P. 1,3-Cyclohexadien-1-Als: Synthesis, Reactivity and Bioactivities. Molecules 2021, 26, 1772. https://doi.org/10.3390/molecules26061772
Tobal IE, Bautista R, Diez D, Garrido NM, García-García P. 1,3-Cyclohexadien-1-Als: Synthesis, Reactivity and Bioactivities. Molecules. 2021; 26(6):1772. https://doi.org/10.3390/molecules26061772
Chicago/Turabian StyleTobal, Ignacio E., Rocío Bautista, David Diez, Narciso M. Garrido, and Pilar García-García. 2021. "1,3-Cyclohexadien-1-Als: Synthesis, Reactivity and Bioactivities" Molecules 26, no. 6: 1772. https://doi.org/10.3390/molecules26061772