Bridging the Chemical Profile and Biological Activities of a New Variety of Agastache foeniculum (Pursh) Kuntze Extracts and Essential Oil
Abstract
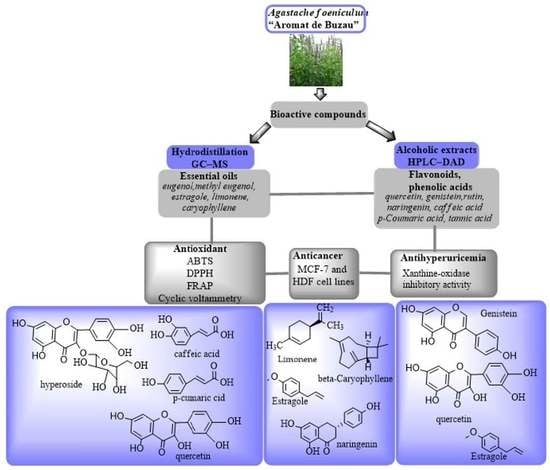
:1. Introduction
2. Results
2.1. Morphological and Structural Characteristics of A. foeniculum AdB Using Confocal Laser Scanning Microscopy (CLSM)
2.2. Profiling of Chemical Compounds in A. foeniculum AdB EO by GC-MS
2.3. Total Phenolic and Flavonoid Contents
2.4. Identification of Bioactive Compounds in the Extracts from A. foeniculum AdB through HPLC-DAD
2.5. In Vitro Antioxidant Activities
2.5.1. 2,2′-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Activity
2.5.2. ABTS [2,2′-Azinobis-(3-ethylbenzothiazoline-6-sulfonic Acid)] Scavenging Activity
2.5.3. FRAP Scavenging Activity
2.6. Electrochemical Evaluation of Antioxidant Capacity by Cyclic Voltammetry
2.7. Xanthine Oxidase (Xo) Inhibitory Activity of the EO and Alcoholic Extracts from A. foeniculum
2.8. Cytotoxic Activity of the EO from A. foeniculum
3. Discussion
4. Materials and Methods
4.1. General
4.2. General Experimental Procedures
4.2.1. Essential Oil Extraction
4.2.2. Gas Chromatography-Mass Spectrometry (GC-MS)
4.2.3. Ultrasound-Assisted Extraction with Solvents (Methanol, Ethanol)
4.2.4. Total Phenolic Content
4.2.5. Total Flavonoid Content
4.2.6. Profiling of Bioactive Compounds by HPLC-DAD
4.3. Antioxidant Assays
4.3.1. In Vitro-Antioxidant Assays
4.3.2. Electrochemical Evaluation of Antioxidant Capacity by Cyclic Voltammetry
4.4. Xanthine Oxidase Inhibitory Activity Assays
4.5. Cytotoxic Activities
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Souto, E.B.; Sampaio, A.C.; Campos, J.R.; Martins-Gomes, C.; Aires, A.; Silva, A.M. Chapter 2—Polyphenols for Skin Cancer: Chemical Properties, Structure-Related Mechanisms of Action and New Delivery Systems. In Bioactive Natural Products; Atta-ur-Rahman, Ed.; Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 63, pp. 21–42. [Google Scholar]
- Khan, T.; Ali, M.; Khan, A.; Nisar, P.; Jan, S.A.; Afridi, S.; Shinwari, Z.K. Anticancer Plants: A Review of the Active Phytochemicals, Applications in Animal Models, and Regulatory Aspects. Biomolecules 2020, 10, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, M.; Zhou, L.; Huang, Z.; Li, B.; Nice, E.C.; Xu, J.; Huang, C. Antioxidant Therapy in Cancer: Rationale and Progress. Antioxidants 2022, 11, 1128. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, U.; Handique, J.G. Chapter 6—Plant Polyphenols as Potent Antioxidants: Highlighting the Mechanism of Antioxidant Activity and Synthesis/Development of Some Polyphenol Conjugates. In Bioactive Natural Products; Atta-ur-Rahman, Ed.; Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2022; Volume 75, pp. 243–266. [Google Scholar]
- Rehman, M.U.; Abdullah; Khan, F.; Niaz, K. Chapter 1—Introduction to Natural Products Analysis. In Recent Advances in Natural Products Analysis; Sanches Silva, A., Nabavi, S.F., Saeedi, M., Nabavi, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–15. ISBN 978-0-12-816455-6. [Google Scholar]
- Xi, X.; Wang, J.; Qin, Y.; You, Y.; Huang, W.; Zhan, J. The Biphasic Effect of Flavonoids on Oxidative Stress and Cell Proliferation in Breast Cancer Cells. Antioxidants 2022, 11, 622. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Nile, A.S.; Keum, Y.S.; Sharma, K. Utilization of Quercetin and Quercetin Glycosides from Onion (Allium cepa L.) Solid Waste as an Antioxidant, Urease and Xanthine Oxidase Inhibitors. Food Chem. 2017, 235, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Shukor, N.A.A.; Ablat, A.; Muhamad, N.A.; Mohamad, J. In Vitro Antioxidant and in Vivo Xanthine Oxidase Inhibitory Activities of Pandanus amaryllifolius in Potassium Oxonate-Induced Hyperuricemic Rats. Int. J. Food Sci. Technol. 2018, 53, 1476–1485. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.Y.; Chang, Y.Y.; Chang, S.T.; Chang, H.T. Xanthine Oxidase Inhibitory Activity and Chemical Composition of Pistacia chinensis Leaf Essential Oil. Pharmaceutics 2022, 14, 1982. [Google Scholar] [CrossRef] [PubMed]
- Vickneson, K.; George, J. Xanthine Oxidoreductase Inhibitors. In Reactive Oxygen Species; Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2021; Volume 264, pp. 205–228. [Google Scholar] [CrossRef]
- Marrelli, M. Medicinal Plants. Plants 2021, 10, 1355. [Google Scholar] [CrossRef]
- Park, C.H.; Yeo, H.J.; Baskar, T.B.; Park, Y.E.; Park, J.S.; Lee, S.Y.; Park, S.U. In Vitro Antioxidant and Antimicrobial Properties of Flower, Leaf, and Stem Extracts of Korean Mint. Antioxidants 2019, 8, 75. [Google Scholar] [CrossRef] [Green Version]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Michela, A.; Montone, I.; Papaianni, M.; Malvano, F.; Capuano, F.; Capparelli, R.; Albanese, D. Lactoferrin, Quercetin, and Hydroxyapatite Act Synergistically against Pseudomonas fluorescens. Int. J. Mol. Sci. 2021, 22, 9247. [Google Scholar]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martínez-Larrañaga, M.R.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M.A. Synthetic Phenolic Antioxidants: Metabolism, Hazards and Mechanism of Action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadaka, A.O.; Sibuyi, N.R.S.; Martin, D.R.; Klein, A.; Madiehe, A.; Meyer, M. Development of Effective Therapeutic Molecule from Natural Sources against Coronavirus Protease. Int. J. Mol. Sci. 2021, 22, 9431. [Google Scholar] [CrossRef] [PubMed]
- Balanescu, F.; Busuioc, A.C.; Botezatu, A.V.D.; Gosav, S.; Avramescu, S.M.; Furdui, B.; Dinica, R.M. Comparative Study of Natural Antioxidants from Glycine max, Anethum graveolens and Pimpinella anisum Seed and Sprout Extracts Obtained by Ultrasound-Assisted Extraction. Separations 2022, 9, 152. [Google Scholar] [CrossRef]
- Karpiński, T.M. Essential Oils of Lamiaceae Family Plants as Antifungals. Biomolecules 2020, 10, 103. [Google Scholar] [CrossRef] [Green Version]
- Lawson, S.K.; Satyal, P.; Setzer, W.N. The Volatile Phytochemistry of Seven Native American Aromatic Medicinal Plants. Plants 2021, 10, 1061. [Google Scholar] [CrossRef]
- Hashemi, M.; Ehsani, A.; Hassani, A.; Afshari, A.; Aminzare, M.; Sahranavard, T.; Azimzadeh, Z. Phytochemical, Antibacterial, Antifungal and Antioxidant Properties of Agastache Foeniculum Essential Oil. J. Chem. Health Risks 2017, 7, 95–104. [Google Scholar]
- Ivanov, I.G.; Vrancheva, R.Z.; Petkova, N.T.; Tumbarski, Y.; Dincheva, I.N.; Badjakov, I.K. Phytochemical Compounds of Anise Hyssop (Agastache foeniculum) and Antibacterial, Antioxidant, and Acetylcholinesterase Inhibitory Properties of Its Essential Oil. J. Appl. Pharm. Sci. 2019, 9, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Lashkari, A.; Najafi, F.; Kavoosi, G.; Niazi, S. Evaluating the In Vitro Anti-Cancer Potential of Estragole from the Essential Oil of Agastache foeniculum [Pursh.] Kuntze. Biocatal. Agric. Biotechnol. 2020, 27, 101727. [Google Scholar] [CrossRef]
- Fuentes-Granados, R.G.; Widrlechner, M.P.; Wilson, L.A. Inheritance Studies of Aromatic Compounds in Agastache Foeniculum (Pursh) Kuntze. J. Essent. Oil Res. 2000, 12, 581–594. [Google Scholar] [CrossRef]
- Talebi, S.F.; Saharkhiz, M.J.; Kermani, M.J.; Sharafi, Y.; Raouf Fard, F. Effect of Different Antimitotic Agents on Polyploid Induction of Anise Hyssop (Agastache foeniculum L.). Caryologia 2017, 70, 184–193. [Google Scholar] [CrossRef]
- Strilbytska, O.M.; Zayachkivska, A.; Koliada, A.; Galeotti, F.; Volpi, N.; Storey, K.B.; Vaiserman, A.; Lushchak, O. Anise Hyssop Agastache foeniculum Increases Lifespan, Stress Resistance, and Metabolism by Affecting Free Radical Processes in Drosophila. Front. Physiol. 2020, 11, 596729. [Google Scholar] [CrossRef] [PubMed]
- Itokawa, H.; Suto, K.; Takeya, K. Structures of Isoagastachoside and Agastachin, New Glucosylflavones Isolated from Agastache rugosa. Chem. Pharm. Bull. 1981, 29, 1777–1779. [Google Scholar] [CrossRef] [Green Version]
- Fathiazad, F.; Hamedeyazdan, S. A Review on Hyssopus officinalis L.: Composition and Biological Activities. Afr. J. Pharm. Pharmacol. 2011, 5, 1959–1966. [Google Scholar] [CrossRef]
- Lee, C.; Kim, H.; Kho, Y. Agastinol and Agastenol, Novel Lignans from Agastache rugosa and Their Evaluation in an Apoptosis Inhibition Assay. J. Nat. Prod. 2002, 65, 414–416. [Google Scholar] [CrossRef]
- Zielińska, S.; Matkowski, A. Phytochemistry and Bioactivity of Aromatic and Medicinal Plants from the Genus Agastache (Lamiaceae). Phytochem. Rev. 2014, 13, 391–416. [Google Scholar] [CrossRef] [Green Version]
- Jovanović, A.A.; Đorđević, V.B.; Zdunić, G.M.; Pljevljakušić, D.S.; Šavikin, K.P.; Gođevac, D.M.; Bugarski, B.M. Optimization of the Extraction Process of Polyphenols from Thymus serpyllum L. Herb Using Maceration, Heat- and Ultrasound-Assisted Techniques. Sep. Purif. Technol. 2017, 179, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Ebadollahi, A. Chemical Constituents and Toxicity of Agastache foeniculum (Pursh) Kuntze Essential Oil against Two Stored-Product Insect Pests. Chil. J. Agric. Res. 2011, 71, 212–217. [Google Scholar] [CrossRef] [Green Version]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.F.; et al. Infectious Disease in an Era of Global Change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef]
- Watkins, K. Emerging Infectious Diseases: A Review. Curr. Emerg. Hosp. Med. Rep. 2018, 6, 86–93. [Google Scholar] [CrossRef]
- Al-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Svitlana, K.; Vladimir, V.; Inna, M. Perspectives Culture of the Lophanthus anisatus Benth. and Peculiarities of Its Ontogenesis in the Conditions of the Lowland Zone of Transcarpathian. Ecol. Evol. Biol. 2020, 5, 29. [Google Scholar] [CrossRef]
- Li, H.Q.; Liu, Q.Z.; Liu, Z.L.; Du, S.S.; Deng, Z.W. Chemical Composition and Nematicidal Activity of Essential Oil of Agastache rugosa against Meloidogyne incognita. Molecules 2013, 18, 4170–4180. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rodríguez, P.; Baquero, L.P.; Larrota, H.R. Flavonoids: Potential Therapeutic Agents by Their Antioxidant Capacity. In Bioactive Compounds; Elsevier Inc.: Amsterdam, The Netherlands, 2018; ISBN 9780128147757. [Google Scholar]
- Pastor-Villaescusa, B.; Rodriguez, E.S.; Rangel-Huerta, O.D. Polyphenols in Obesity and Metabolic Syndrome. In Obesity Oxidative Stress and Dietary Antioxidants; Academic Press: Cambridge, MA, USA, 2018; pp. 213–239. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Tratrat, C.; Chandrashekharappa, S.; Attimarad, M.; Sreeharsha, N.; Nair, A.B.; Pottathil, S.; Venugopala, R.; Al-Attraqchi, O.H.A.; Morsy, M.A.; et al. Anti-Tubercular Potency and Computationally- Assessed Drug-Likeness and Toxicology of Diversely Substituted Indolizines. Indian J. Pharm. Educ. Res. 2019, 53, 545–552. [Google Scholar] [CrossRef] [Green Version]
- Charles, D.J.; Simon, J.E.; Widrlechner, M.P. Characterization of Essential Oil of Agastache Species. J. Agric. Food Chem. 1991, 39, 1946–1949. [Google Scholar] [CrossRef] [Green Version]
- Mazza, G.; Kiehn, F.A. Essential Oil of Agastache foeniculum, a Potential Source of Methyl Chavicol. J. Essent. Oil Res. 1992, 4, 295–299. [Google Scholar] [CrossRef]
- Skakovskii, E.D.; Kiselev, W.P.; Tychinskaya, L.Y.; Schutova, A.G.; Gonsharova, L.W.; Spiridowish, E.W.; Bovdey, N.A.; Kiselev, P.A.; Gaidukevich, O.A. Characterization of the Essential Oil of Agastache rugosa by NMR Spectroscopy. J. Appl. Spectrosc. 2010, 77, 329–334. [Google Scholar] [CrossRef]
- Bayguinov, P.O.; Oakley, D.M.; Shih, C.C.; Geanon, D.J.; Joens, M.S.; Fitzpatrick, J.A.J. Modern Laser Scanning Confocal Microscopy. Curr. Protoc. Cytom. 2018, 85, 1–17. [Google Scholar] [CrossRef]
- Borges, A.; José, H.; Homem, V.; Simões, M. Comparison of Techniques and Solvents on the Antimicrobial and Antioxidant Potential of Extracts from Acacia dealbata and Olea europaea. Antibiotics 2020, 9, 48. [Google Scholar] [CrossRef]
- Palma-Tenango, M.; Sánchez-Fernández, R.E.; Soto-Hernández, M. A Systematic Approach to Agastache mexicana Research: Biology, Agronomy, Phytochemistry, and Bioactivity. Molecules 2021, 26, 3751. [Google Scholar] [CrossRef]
- Igbinosa, O.O.; Igbinosa, I.H.; Chigor, V.N.; Uzunuigbe, O.E.; Oyedemi, S.O.; Odjadjare, E.E.; Okoh, A.I.; Igbinosa, E.O. Polyphenolic Contents and Antioxidant Potential of Stem Bark Extracts from Jatropha curcas (Linn). Int. J. Mol. Sci. 2011, 12, 2958–2971. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.G.d.F.; Hilário, F.; Vilegas, W.; dos Santos, L.C.; Brunetti, I.L.; Sotomayor, C.E.; Bauab, T.M. Correlation among Antioxidant, Antimicrobial, Hemolytic, and Antiproliferative Properties of Leiothrix spiralis Leaves Extract. Int. J. Mol. Sci. 2012, 13, 9260–9277. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Leem, E.; Lee, J.M.; Kim, S.R. Control of Reactive Oxygen Species for the Prevention of Parkinson’s Disease: The Possible Application of Flavonoids. Antioxidants 2020, 9, 583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, R.; Zhang, G.; Gong, D. Mechanistic Insights into the Inhibition of Quercetin on Xanthine Oxidase. Int. J. Biol. Macromol. 2018, 112, 405–412. [Google Scholar] [CrossRef]
- Liu, D.; Wang, D.; Yang, W.; Meng, D. Potential Anti-Gout Constituents as Xanthine Oxidase Inhibitor from the Fruits of Stauntonia brachyanthera. Bioorg. Med. Chem. 2017, 25, 3562–3566. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.; Ademiluyi, A.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Lima, C.C.; de Holanda-Angelin-Alves, C.M.; Pereira-Gonçalves, Á.; Kennedy-Feitosa, E.; Evangelista-Costa, E.; Bezerra, M.A.C.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H. Antispasmodic Effects of the Essential Oil of Croton Zehnteneri, Anethole, and Estragole, on Tracheal Smooth Muscle. Heliyon 2020, 6, e05445. [Google Scholar] [CrossRef]
- European Commission Regulation (EC) No 1334/2008 on Flavourings. Off. J. Eur. Union 2008, L 354/34, 34–50.
- Eisenbrand, G.; Cohen, S.M.; Fukushima, S.; Gooderham, N.J.; Guengerich, F.P.; Hecht, S.S.; Rietjens, I.M.C.M.; Rosol, T.J.; Davidsen, J.M.; Harman, C.L.; et al. FEMA GRAS Assessment of Natural Flavor Complexes: Eucalyptus Oil and Other Cyclic Ether-Containing Flavoring Ingredients. Food Chem. Toxicol. 2021, 155, 112357. [Google Scholar] [CrossRef]
- Bristol, D.W. NTP 3-Month Toxicity Studies of Estragole Administered by Gavage to F344/N Rats and B6C3F1 Mice. Toxic Rep. Ser. 2011, 82, 1–111. [Google Scholar]
- Gori, L.; Gallo, E.; Mascherini, V.; Mugelli, A.; Vannacci, A.; Firenzuoli, F. Can Estragole in Fennel Seed Decoctions Really Be Considered a Danger for Human Health? A Fennel Safety Update. Evid.-Based Complement. Altern. Med. 2012, 2012, 860542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebadollahi, A.; Safaralizadeh, M.; Pourmirza, A.; Gheibi, S. Toxicity of Essential Oil of Agastache foeniculum (Pursh) Kuntze to Oryzaephilus surinamensis L. and Lasioderma serricorne F. J. Plant Prot. Res. 2010, 50, 215–219. [Google Scholar] [CrossRef]
- NIST/EPA/NIH Mass Spectral Database; SRD Program; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2011.
- Gonfa, T.; Teketle, S.; Kiros, T. Effect of Extraction Solvent on Qualitative and Quantitative Analysis of Major Phyto-Constituents and in-Vitro Antioxidant Activity Evaluation of Cadaba rotundifolia Forssk Leaf Extracts. Cogent Food Agric. 2020, 6, 1853867. [Google Scholar] [CrossRef]
- Balanescu, F.; Mihaila, M.D.I.; Cârâc, G.; Furdui, B.; Vînătoru, C.; Avramescu, S.M.; Lisa, E.L.; Cudalbeanu, M.; Dinica, R.M. Flavonoid Profiles of Two New Approved Romanian Ocimum Hybrids. Molecules 2020, 25, 4573. [Google Scholar] [CrossRef]
- Cudalbeanu, M.; Ghinea, I.O.; Furdui, B.; Dah-Nouvlessounon, D.; Raclea, R.; Costache, T.; Cucolea, I.E.; Urlan, F.; Dinica, R.M. Exploring New Antioxidant and Mineral Compounds from Nymphaea alba Wild-Grown in Danube Delta Biosphere. Molecules 2018, 23, 1247. [Google Scholar] [CrossRef] [Green Version]
- Chisté, R.C.; Mercadante, A.Z. Identification and Quantification, by HPLC-DAD-MS/MS, of Carotenoids and Phenolic Compounds from the Amazonian Fruit Caryocar villosum. J. Agric. Food Chem. 2012, 60, 5884–5892. [Google Scholar] [CrossRef]
- Busuioc, A.C.; Botezatu, A.V.D.; Furdui, B.; Vinatoru, C.; Maggi, F.; Caprioli, G.; Dinica, R.M. Comparative Study of the Chemical Compositions and Antioxidant Activities of Fresh Juices from Romanian Cucurbitaceae Varieties. Molecules 2020, 25, 5468. [Google Scholar] [CrossRef]
- Wandjou, J.G.N.; Mevi, S.; Sagratini, G.; Vittori, S.; Dall’acqua, S.; Caprioli, G.; Lupidi, G.; Mombelli, G.; Arpini, S.; Allegrini, P.; et al. Antioxidant and Enzyme Inhibitory Properties of the Polyphenolic-Rich Extract from an Ancient Apple Variety of Central Italy (Mela Rosa Dei Monti Sibillini). Plants 2020, 9, 9. [Google Scholar] [CrossRef] [Green Version]
- Cudalbeanu, M.; Furdui, B.; Cârâc, G.; Barbu, V.; Iancu, A.V.; Marques, F.; Leitão, J.H.; Sousa, S.A.; Dinica, R.M. Antifungal, Antitumoral and Antioxidant Potential of the Danube Delta Nymphaea Alba Extracts. Antibiotics 2020, 9, 7. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Hu, J.; Hu, D.; Yang, X. A Role of Gallic Acid in Oxidative Damage Diseases: A Comprehensive Review. Nat. Prod. Commun. 2019, 14. [Google Scholar] [CrossRef] [Green Version]
- Rivero-Cruz, J.F.; Granados-Pineda, J.; Pedraza-Chaverri, J.; Pérez-Rojas, J.M.; Kumar-Passari, A.; Diaz-Ruiz, G.; Rivero-Cruz, B.E. Phytochemical Constituents, Antioxidant, Cytotoxic, and Antimicrobial Activities of the Ethanolic Extract of Mexican Brown Propolis. Antioxidants 2020, 9, 70. [Google Scholar] [CrossRef] [PubMed]











| No. | Rt (min.) | Constituent | m/z | Molecular Formula & Molecular Weight | Concentration (%) |
|---|---|---|---|---|---|
| 1 | 8.16 |  Limonene | 136 | C10H16(MW: 136.23) | 2.91 ± 0.65 b |
| 2 | 10.82 |  Methyl chavicol (Estragole) | 148 | C10H12O(MW: 148.20) | 94.89 ± 1.02 a |
| 3 | 12.07 | Unknown | 0.40 ± 0.28 d | ||
| 4 | 12.76 | Unknown | 0.04 ± 0.02 f | ||
| 5 | 12.97 |  Eugenol | 164 | C10H12O2(MW: 164.20) | 0.01 ± 0.01 g |
| 6 | 13.18 |  Methyl isoeugenol | 178 | C11H14O2(MW: 178.23) | 0.04 ± 0.01 f |
| 7 | 13.57 |  Methyl eugenol | 178 | C11H14O2(MW: 178.23) | 0.73 ± 0.04 c |
| 8 | 13.98 |  Caryophyllene | 204 | C15H24(MW: 204.35) | 0.74 ± 0.02 c |
| 9 | 14.75 |  Germacrene D | 204 | C15H24(MW: 204.35) | 0.23 ± 07 e |
| Samples | Extraction Yield (%) | TPC (mg GAE/g DW) | TFC (mg QE/g DW) |
|---|---|---|---|
| MeOH | 11.062 ± 0.945 a | 485.084 ± 0.052 a | 367.32 ± 0.008 a |
| EtOH | 7.211 ± 0.686 b | 403.918 ± 0.057 b | 355.94 ± 0.007 b |
| Chemical Name | Chemical Structure | Content (µg/g dw) | |
|---|---|---|---|
| MeOH | EtOH | ||
| Phenolic Acids | |||
| p-Coumaric acid | C9H8O3 | 62.079 ± 0.436 a | 8.982 ± 0.093 b |
| Caffeic acid | C9H8O4 | 59.014 ± 0.103 a | 31.320 ± 0.145 b |
| Tannins | |||
| Tannic acid | C76H52O46 | 72.061 ± 0.077 a | - |
| Flavonols | |||
| Rutin | C27H30O16 | 76.409 ± 0.100 a | 56.701 ± 0.111 b |
| Quercetin | C15H10O7 | 1073.637 ± 0.130 a | 704.148 ± 0.150 b |
| Hyperoside | C21H20O12 | 98.693 ± 0.190 a | 58.892 ± 0.105 b |
| Flavanones | |||
| Naringenin | C15H12O5 | 43.683 ± 0.114 a | 30.242 ± 0.121 a |
| Isoflavones | |||
| Genistein | C15H10O5 | 3171.823 ± 0.218 a | 2229.999 ± 0.256 b |
| Sample | DPPH IC50 Value | ABTS IC50 Value | FRAP (µM Fe(II) Equivalents/g of Extract) | Unit of Measurement |
|---|---|---|---|---|
| MeOH | 76.368 ± 0.002 b | 98.274 ± 0.002 b | 45.721 ± 0.014 a | µg/mL |
| EtOH | 94.986 ± 0.002 a | 244.261 ± 0.003 a | 39.483 ± 0.017 a | µg/mL |
| EO | 12.943 ± 0.001 c | 0.3356 ± 0.002 d | µg/mL | |
| Trolox | 32.562 ± 0.002 c | µM | ||
| Gallic acid | 15.614 ± 0.012 d | µg/mL |
| TPC a | TFC b | DPPH•c | ABTS•+ d | FRAP e | |
|---|---|---|---|---|---|
| TPC | 1 | ||||
| TFC | 0.9087 * | 1 | |||
| DPPH• | 0.9373 * | 0.9972 ** | 1 | ||
| ABTS•+ | 0.9720 ** | 0.9813 ** | 0.9929 ** | 1 | |
| FRAP | 0.7356 | 0.9275 * | 0.9057 * | 0.8600 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bălănescu, F.; Botezatu, A.V.; Marques, F.; Busuioc, A.; Marincaş, O.; Vînătoru, C.; Cârâc, G.; Furdui, B.; Dinica, R.M. Bridging the Chemical Profile and Biological Activities of a New Variety of Agastache foeniculum (Pursh) Kuntze Extracts and Essential Oil. Int. J. Mol. Sci. 2023, 24, 828. https://doi.org/10.3390/ijms24010828
Bălănescu F, Botezatu AV, Marques F, Busuioc A, Marincaş O, Vînătoru C, Cârâc G, Furdui B, Dinica RM. Bridging the Chemical Profile and Biological Activities of a New Variety of Agastache foeniculum (Pursh) Kuntze Extracts and Essential Oil. International Journal of Molecular Sciences. 2023; 24(1):828. https://doi.org/10.3390/ijms24010828
Chicago/Turabian StyleBălănescu, Fănică, Andreea Veronica Botezatu, Fernanda Marques, Anna Busuioc, Olivian Marincaş, Costel Vînătoru, Geta Cârâc, Bianca Furdui, and Rodica Mihaela Dinica. 2023. "Bridging the Chemical Profile and Biological Activities of a New Variety of Agastache foeniculum (Pursh) Kuntze Extracts and Essential Oil" International Journal of Molecular Sciences 24, no. 1: 828. https://doi.org/10.3390/ijms24010828