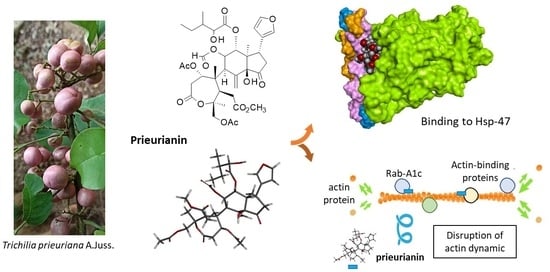
Insights into the Mechanism of Action of the Degraded Limonoid Prieurianin
Abstract
:1. Introduction
2. Prieurianin-Type Limonoids: Structure and Origins
3. Bioactivities of Prieurianin and Analogs
4. Potential Molecular Targets of Prieurianin and Analogs

5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Roy, A.; Saraf, S. Limonoids: Overview of significant bioactive triterpenes distributed in plants kingdom. Biol. Pharm. Bull. 2006, 29, 191–201. [Google Scholar] [CrossRef]
- Luo, J.; Sun, Y.; Li, Q.; Kong, L. Research progress of meliaceous limonoids from 2011 to 2021. Nat. Prod. Rep. 2022, 39, 1325–1365. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Qin, D. Classification of Diverse Novel Limonoids. In Novel Plant Natural Product Skeletons; Springer: Singapore, 2024. [Google Scholar] [CrossRef]
- Gualdani, R.; Cavalluzzi, M.M.; Lentini, G.; Habtemariam, S. The Chemistry and Pharmacology of Citrus Limonoids. Molecules 2016, 21, 1530. [Google Scholar] [CrossRef]
- Arora, S.; Mohanpuria, P.; Sidhu, G.S. Citrus limonoids: Mechanism, function and its metabolic engineering for human health. Fruits 2018, 73, 158–173. [Google Scholar] [CrossRef]
- Shi, Y.S.; Zhang, Y.; Li, H.T.; Wu, C.H.; El-Seedi, H.R.; Ye, W.K.; Wang, Z.W.; Li, C.B.; Zhang, X.F.; Kai, G.Y. Limonoids from Citrus: Chemistry, anti-tumor potential, and other Bioactivities. J. Function. Food 2020, 75, 104213. [Google Scholar] [CrossRef]
- Hilmayanti, E.; Nurlelasari Supratman, U.; Kabayama, K.; Shimoyama, A.; Fukase, K. Limonoids with anti-inflammatory activity: A review. Phytochemistry 2022, 204, 113469. [Google Scholar] [CrossRef]
- Zheng, W.; Yang, S.; Chen, X. The pharmacological and pharmacokinetic properties of obacunone from citrus fruits: A comprehensive narrative review. Fitoterapia 2023, 169, 105569. [Google Scholar] [CrossRef]
- Zhou, Z.; Yan, Y.; Li, H.; Feng, Y.; Huang, C.; Fan, S. Nomilin and Its Analogues in Citrus Fruits: A Review of Its Health Promotion Effects and Potential Application in Medicine. Molecules 2022, 29, 269. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, L.; Hu, M.; Yang, Y.; Ma, Q.; Chen, J. Network Pharmacology to Reveal the Molecular Mechanisms of Rutaceous Plant-derived Limonin Ameliorating Non-alcoholic Steatohepatitis. Crit. Rev. Immunol. 2023, 43, 11–23. [Google Scholar] [CrossRef]
- Liang, H.; Liu, G.; Fan, Q.; Nie, Z.; Xie, S.; Zhang, R. Limonin, a novel AMPK activator, protects against LPS-induced acute lung injury. Int. Immunopharmacol. 2023, 122, 110678. [Google Scholar] [CrossRef]
- Zhou, X.; Xiang, Y.; Li, D.; Zhong, M.; Hong, X.; Gui, Y.; Min, W.; Chen, Y.; Zeng, X.; Zhu, H.; et al. Limonin, a natural ERK2 agonist, protects against ischemic acute kidney injury. Int. J. Biol. Sci. 2023, 19, 2860–2878. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, C.; Luo, T.; Wang, J.; Tang, Y.; Chen, Z.; Yu, L. Limonin: A Review of Its Pharmacology, Toxicity, and Pharmacokinetics. Molecules 2019, 24, 3679. [Google Scholar] [CrossRef]
- Furiassi, L.; Tonogai, E.J.; Hergenrother, P.J. Limonin as a Starting Point for the Construction of Compounds with High Scaffold Diversity. Angew. Chem. Int. Ed. Engl. 2021, 60, 16119–16128. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, H.; De La Peña, R.; Stephenson, M.J.; Thimmappa, R.; Vincent, J.L.; Sattely, E.S.; Osbourn, A. Identification of key enzymes responsible for protolimonoid biosynthesis in plants: Opening the door to azadirachtin production. Proc. Natl. Acad. Sci. USA 2019, 116, 17096–17104. [Google Scholar] [CrossRef] [PubMed]
- Aarthy, T.; Mulani, F.A.; Pandreka, A.; Kumar, A.; Nandikol, S.S.; Haldar, S.; Thulasiram, H.V. Tracing the biosynthetic origin of limonoids and their functional groups through stable isotope labeling and inhibition in neem tree (Azadirachta indica) cell suspension. BMC Plant Biol. 2018, 18, 230. [Google Scholar] [CrossRef]
- De La Peña, R.; Hodgson, H.; Liu, J.C.; Stephenson, M.J.; Martin, A.C.; Owen, C.; Harkess, A.; Leebens-Mack, J.; Jimenez, L.E.; Osbourn, A.; et al. Complex scaffold remodeling in plant triterpene biosynthesis. Science 2023, 379, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.G.; Luo, X.D. Meliaceous Limonoids: Chemistry and Biological Activities. Chem. Rev. 2011, 111, 7437–7522. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, H. Recent progress in the chemistry and biology of limonoids. RSC Adv. 2017, 7, 35191. [Google Scholar] [CrossRef]
- Durán-Peña, M.J.; Botubol-Ares, J.M.; Collado, I.G.; Hernandez-Galan, R. Degraded limonoids: Biologically active limonoid fragments re-enhancing interest in Meliaceae and Rutaceae sources. Phytochem. Rev. 2023, 22, 695–741. [Google Scholar] [CrossRef]
- Ferrera-Suanzes, M.; Prieto, V.; Medina-Olivera, A.J.; Botubol-Ares, J.M.; Galán-Sánchez, F.; Rodríguez-Iglesias, M.A.; Hernández-Galán, R.; Durán-Peña, M.J. Synthesis of Degraded Limonoid Analogs as New Antibacterial Scaffolds against Staphylococcus aureus. Antibiotics 2020, 9, 488. [Google Scholar] [CrossRef]
- Bailly, C.; Vergoten, G. Fraxinellone: From pesticidal control to cancer treatment. Pestic. Biochem. Physiol. 2020, 168, 104624. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, R.; Xu, H. New Insecticidal Agents from Halogenation/Acylation of the Furyl-Ring of Fraxinellone. Sci. Rep. 2016, 6, 35321. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Fan, J.; Zhang, Q.; Bao, C.; Liu, Z.; Yang, R. Turning natural products into insecticide candidates: Design and semisynthesis of novel fraxinellone-based N-(1,3-thiazol-2-yl)carboxamides against two crop-threatening insect pests. Bioorg. Med. Chem. Lett. 2019, 29, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.J.; Dong, Q.M.; Wang, M.R.; Tang, J.J. Semi-Synthesis of C-Ring Cyclopropyl Analogues of Fraxinellone and Their Insecticidal Activity Against Mythimna separata Walker. Molecules 2020, 25, 1109. [Google Scholar] [CrossRef] [PubMed]
- Happi, G.M.; Nangmo, P.K.; Dzouemo, L.C.; Kache, S.F.; Kouam, A.D.K.; Wansi, J.D. Contribution of Meliaceous plants in furnishing lead compounds for antiplasmodial and insecticidal drug development. J. Ethnopharmacol. 2022, 285, 114906. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Yang, S.; Huang, J.; Zhou, L. Insecticidal Triterpenes in Meliaceae: Plant Species, Molecules and Activities: Part I (Aphanamixis-Chukrasia). Int. J. Mol. Sci. 2021, 22, 13262. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Bi, X.; Zhou, L.; Huang, J. Insecticidal Triterpenes in Meliaceae: Plant Species, Molecules, and Activities: Part II (Cipadessa, Melia). Int. J. Mol. Sci. 2022, 23, 5329. [Google Scholar] [CrossRef]
- Bevan, C.W.L.; Ekong, D.E.U.; Taylor, D.A.H. Extractives from West African Members of the Family Meliaceae. Nature 1965, 206, 1323–1325. [Google Scholar] [CrossRef]
- Trichilia prieuriana. Available online: https://tropical.theferns.info/viewtropical.php?id=Trichilia+prieuriana (accessed on 18 February 2024).
- Oyedeji-Amusa, M.O.; Sadgrove, N.J.; Van Wyk, B.E. The Ethnobotany and Chemistry of South African Meliaceae: A Review. Plants 2021, 10, 1796. [Google Scholar] [CrossRef]
- Kangbéto Bidossessi, R.; Attakpa Sèlidji, E.; Guinnin, F.; Sénou, M.; Lagnika, L. Toxicological assessment of ethanolic extracts of Annona senegalensis and Trichilia prieureana in the treatment of type 2 diabetes in Benin. J. Physiol. Pathophysiol. 2022, 13, 27–35. [Google Scholar] [CrossRef]
- Gullo, V.P.; Miura, I.; Akanishi, K. Structure of Prieurianin, a Complex Tetranortriterpenoid; Nuclear Magnetic Resonance Analysis at Nonambient Temperatures and X-Ray Structure Determination. J. Chem. Soc. Chem. Commun. 1975, 9, 345–346. [Google Scholar] [CrossRef]
- Pagna, J.I.M.; Mbekou, I.M.K.; Tsamo, A.T.; Mkounga, P.; Frese, M.; Stammler, H.G.; Fekam, F.B.; Lenta, B.N.; Sewald, N.; Nkengfack, A.E. Antibacterial activity of some chemical constituents from Trichilia prieuriana (Meliaceae). Z. Naturforschung B 2021, 76, 439–446. [Google Scholar] [CrossRef]
- Resetar, M.; Tietcheu Galani, B.R.; Tsamo, A.T.; Chen, Y.; Schachner, D.; Stolzlechner, S.; Mawouma Pagna, J.I.; Beniddir, M.A.; Kirchmair, J.; Dirsch, V.M. Flindissone, a Limonoid Isolated from Trichilia prieuriana, Is an LXR Agonist. J. Nat. Prod. 2023, 86, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Olugbade, T.A. Tetracyclic triterpenoids from Trichilia prieuriana leaves. Phytochemistry 1991, 30, 698–700. [Google Scholar] [CrossRef]
- Olugbade, T.A.; Adesanya, S.A. Prieurianoside, a protolimonoid glucoside from the leaves of Trichilia prieuriana. Phytochemistry 2000, 54, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Lukacova, V.; Polonsky, J.; Moretti, C. Isolation and structure of 14,15β-epoxyprieurianin from the South American tree Guarea guidona. J. Nat. Prod. 1982, 45, 288–294. [Google Scholar] [CrossRef]
- Sarker, S.D.; Savchenko, T.; Whiting, P.; Sik, V.; Dinan, L. Two limonoids from Turraea obtusifolia (Meliaceae), prieurianin and rohitukin, antagonise 20-hydroxyecdysone action in a Drosophila cell line. Arch. Insect. Biochem. Physiol. 1997, 35, 211–217. [Google Scholar] [CrossRef]
- MacLachlan, L.K.; Taylor, D.A.H. Limonoids from Nymania capensis. Phytochemistry 1982, 21, 1701–1703. [Google Scholar] [CrossRef]
- Lin, C.J.; Lo, I.W.; Lin, Y.C.; Chen, S.Y.; Chien, C.T.; Kuo, Y.H.; Hwang, T.L.; Liou, S.S.; Shen, Y.C. Tetranortriterpenes and Limonoids from the Roots of Aphanamixis polystachya. Molecules 2016, 21, 1167. [Google Scholar] [CrossRef]
- Koul, O.; Daniewski, W.M.; Multani, J.S.; Gumulka, M.; Singh, G. Antifeedant effects of the limonoids from Entandrophragma candolei (Meliaceae) on the gram pod borer, Helicoverpa armigera (Lepidoptera: Noctuidae). J. Agric. Food Chem. 2003, 51, 7271–7275. [Google Scholar] [CrossRef]
- Happi, G.M.; Mouthe Kemayou, G.P.; Stammler, H.G.; Neumann, B.; Ismail, M.; Kouam, S.F.; Wansi, J.D.; Tchouankeu, J.C.; Frese, M.; Lenta, B.N.; et al. Three phragmalin-type limonoids orthoesters and the structure of odoratone isolated from the bark of Entandrophragma candollei (Meliaceae). Phytochemistry 2021, 181, 112537. [Google Scholar]
- Mutombo Mianda, S.; Moyo, P.; Maboane, S.; Birkholtz, L.M.; Maharaj, V.J. Phytoconstituents from Turraea obtusifolia and their antiplasmodial activity. Nat. Prod. Res. 2023, 1–13. [Google Scholar] [CrossRef]
- Brown, D.A.; Taylor, D.A.H. Limonoid extractives from Aphanamixis polystachia. Phytochemistry 1978, 17, 1995–1999. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.S.; Wang, X.B.; Gu, Y.C.; Wei, D.D.; Guo, C.; Yang, M.H.; Kong, L.Y. Limonoids from the fruits of Aphanamixis polystachya (Meliaceae) and their biological activities. J. Agric. Food Chem. 2013, 61, 2171–2182. [Google Scholar] [CrossRef]
- Fattah, I.M.T.R. Biodiesel production, characterization, diesel engine performance, and emission characteristics of methyl esters from Aphanamixis polystachya oil of Bangladesh. Energy Convers. Manag. 2015, 91, 149–157. [Google Scholar]
- Ifteqar, S.; Sultana, R.; Banik, S.; Rahman, A.F.M.M. Production and Characterization of Biodiesel from Aphanamixis polystachya Seed Oil. Dhaka Univ. J. Sci. 2020, 68, 129–136. [Google Scholar] [CrossRef]
- Ahmmed, R.; Hasan, I.; Mortuza, G.; Ismail, M. Preparation and physico-chemcial properties evaluation of biodiesel from Pithraj (Aphanamixis polystachya) seeds available in Bangladesh. J. Chem. Engineer. Res. Bull. 2020, 22, 43–48. [Google Scholar]
- Musza, L.L.; Killar, L.M.; Speight, P.; McElhiney, S.; Barrow, C.J.; Gillum, A.M.; Cooper, R. Potent New Cell Adhesion Inhibitory Compounds from the Root of Trichilia rubra. Tetrahedron 1994, 50, 11369–11378. [Google Scholar] [CrossRef]
- Cai, J.Y.; Chen, D.Z.; Luo, S.H.; Kong, N.C.; Zhang, Y.; Di, Y.T.; Zhang, Q.; Hua, J.; Jing, S.X.; Li, S.L.; et al. Limonoids from Aphanamixis polystachya and their antifeedant activity. J. Nat. Prod. 2014, 77, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xue, S.; Huang, W.; Wang, C.C.; Cui, Z.; Luo, J.; Kong, L. Diverse prieurianin-type limonoids with oxygen-bridged caged skeletons from two Aphanamixis species: Discovery and biomimetic conversion. Org. Chem. Front. 2021, 8, 566–571. [Google Scholar] [CrossRef]
- Xie, Y.S.; Isman, M.B.; Gunning, P.; Mackinnon, S.; Arnason, J.T.; Taylor, D.R.; Sanchez, P.; Hasbun, C.; Towers, G.H.N. Biological Activity of Extracts of Trichilia Species and the Limonoid Hirtin Against Lepidopteran Larvae. Biochem. System. Ecol. 1994, 22, 129–136. [Google Scholar] [CrossRef]
- Passos, M.; Nogueira, T.S.R.; Azevedo, O.; Vieira, M.G.C.; da silva Terra, W.; Braz-Filho, R.; Vieira, I.J.C. Limonoids from the genus Trichilia and biological activities: Review. Phytochem. Rev. 2021, 20, 1055–1086. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.S.; Gu, Y.C.; Wang, X.B.; Kong, L.Y. Diverse prieurianin-type limonoid derivatives from the fruits of Aphanamixis grandifolia and their absolute configuration Determination. Tetrahedron 2014, 70, 6594–6606. [Google Scholar] [CrossRef]
- Chatterjee, A.; Kundu, A.B.; Chakrabortty, T.; Chandrasekharan, S. Extractives of Aphanamixis polystachya Wall (Parker). The structures and stereochemistry of aphanamixin and aphanamixinin. Tetrahedron 1970, 26, 1859–1867. [Google Scholar] [CrossRef]
- Agnihotri, V.K.; Srivastava, S.D.; Srivastava, S.K. A New Limonoid, Amoorinin, from the Stem Bark of Amoora rohituka. Planta Med. 1987, 53, 298–299. [Google Scholar] [CrossRef]
- Cai, J.Y.; Zhang, Y.; Luo, S.H.; Chen, D.Z.; Tang, G.H.; Yuan, C.M.; Di, Y.T.; Li, S.H.; Hao, X.J.; He, H.P. Aphanamixoid A, a potent defensive limonoid, with a new carbon skeleton from Aphanamixis polystachya. Org. Lett. 2012, 14, 2524–7252. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.B.; Wei, D.D.; Luo, J.G.; Kuo, J.; Yang, M.H.; Kong, L.Y. Aphapolynins A and B, two new limonoids from the fruits of Aphanamixis polystachya. Tetrahedron Lett. 2011, 52, 2590–2593. [Google Scholar] [CrossRef]
- Yu, J.H.; Wang, G.C.; Han, Y.S.; Wu, Y.; Wainberg, M.A.; Yue, J.M. Limonoids with Anti-HIV Activity from Cipadessa cinerascens. J. Nat. Prod. 2015, 78, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Han, M.L.; Zhao, J.X.; Liu, H.C.; Ni, G.; Ding, J.; Yang, S.P.; Yue, J.M. Limonoids and Triterpenoids from Dysoxylum mollissimum var. glaberrimum. J. Nat. Prod. 2015, 78, 754–761. [Google Scholar] [CrossRef]
- Luo, X.D.; Wu, S.H.; Ma, Y.B.; Wu, D.G. Prieurianin-type tetranortriterpenoids from the bark of Dysoxylum hainanense. Heterocycles 2000, 53, 2225–2232. [Google Scholar] [CrossRef]
- Adul, G.O.; Bentley, M.D.; Benson, B.W.; Huang, F.Y.; Gelbaum, L.; Hassanali, A. Two new prieurianin-class limonoids from Turraea mombasana. J. Nat. Prod. 1993, 56, 1414–1417. [Google Scholar] [CrossRef]
- Ge, H.Y.; Liu, K.X.; Zhang, J.X.; Mu, S.Z.; Hao, X.J. The Limonoids and Their Antitobacco Mosaic Virus (TMV) Activities from Munronia unifoliolata Oliv. J Agric Food Chem. 2012, 60, 4289–4295. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, J.X.; Huang, T.; Mao, X.Y.; Gu, W.; He, H.P.; Di, Y.T.; Li, S.L.; Chen, D.Z.; Zhang, Y.; et al. Bioactive Limonoid Constituents of Munronia henryi. J. Nat. Prod. 2015, 78, 811–821. [Google Scholar] [CrossRef]
- Yan, Y.; Yuan, C.M.; Di, Y.T.; Huang, T.; Fan, Y.M.; Ma, Y.; Zhang, J.X.; Hao, X.J. Limonoids from Munronia henryi and their anti-tobacco mosaic virus activity. Fitoterapia 2015, 107, 29–35. [Google Scholar] [CrossRef]
- Yan, Y.; Tang, L.; Hu, J.; Wang, J.; Adelakun, T.A.; Yang, D.; Di, Y.; Zhang, Y.; Hao, X. Munronin O, a potential activator for plant resistance. Pestic. Biochem. Physiol. 2018, 146, 13–18. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, D.; Zhang, X.; Peng, M.; Yan, X.; Guo, Y.; Jia, M.; Zhou, J.; Tang, L.; Hao, X. Anti-TMV activity and effects of three prieurianin-type limonoids from Munronia henryi. Pestic. Biochem. Physiol. 2022, 184, 105108. [Google Scholar] [CrossRef]
- Yang, X.R.; Tanaka, N.; Tsuji, D.; Lu, F.L.; Yan, X.J.; Itoh, K.; Li, D.P.; Kashiwada, Y. Limonoids from the aerial parts of Munronia pinnata. Tetrahedron 2019, 75, 130779. [Google Scholar] [CrossRef]
- Rodriguez-Hahn, L.; Cardenas, J.; Arenas, C. Trichavensin, a prieurianin derivative from Trichilia havannensis. Phytochemistry 1996, 43, 457–459. [Google Scholar] [CrossRef]
- Limachi, I.; Gonzalez-Ramirez, M.; Manner, S.; Ticona, J.C.; Salamanca, E.; Gimenez, A.; Sterner, O. Trichilianones A-D, Novel Cyclopropane-Type Limonoids from Trichilia adolfi. Molecules 2021, 26, 1019. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ramirez, M.; Limachi, I.; Manner, S.; Ticona, J.C.; Salamanca, E.; Gimenez, A.; Sterner, O. Trichilones A-E: New Limonoids from Trichilia adolfi. Molecules 2021, 26, 3070. [Google Scholar] [CrossRef] [PubMed]
- Tsopgni, W.D.T.; Happi, G.M.; Stammler, H.-G.; Neumann, B.; Mbobda, A.S.W.; Kouam, S.F.; Frese, M.; Azébazé, A.G.B.; Lenta, B.N.; Sewald, N. Chemical constituents from the bark of the Cameroonian mahogany Trichilia emetica Vahl (Meliaceae). Phytochem. Lett. 2019, 33, 49–54. [Google Scholar] [CrossRef]
- Ogbuewu, I.P.; Odoemenam, V.U.; Obikaonu, H.O.; Opara, M.N.; Emenalom, O.O.; Uchegbu, M.C.; Okoli, I.C.; Esonu, B.O.; Iloeje, M.U. The growing importance of neen (Azadirachta indica A. Juss) in agriculture, industry, medicine and environment: A review. Res. J. Med. Plant 2011, 5, 230–245. [Google Scholar]
- Sarkar, S.; Singh, R.P.; Bhattacharya, G. Exploring the role of Azadirachta indica (neem) and its active compounds in the regulation of biological pathways: An update on molecular approach. 3 Biotech 2021, 11, 178. [Google Scholar] [CrossRef]
- Nagini, S.; Palrasu, M.; Bishayee, A. Limonoids from neem (Azadirachta indica A. Juss.) are potential anticancer drug candidates. Med. Res. Rev. 2024, 44, 457–496. [Google Scholar] [CrossRef]
- Connolly, J.D.; Okorie, D.A.; de Wit, L.D.; Taylor, D.A.H. Structure of dregeanin and rohitukin, limonoids from the subfamily Melioideae of the family Meliaceae. An unusually high absorption frequency for a six-membered lactone ring. J. Chem. Soc. Chem. Commun. 1976, 22, 909. [Google Scholar] [CrossRef]
- Connolly, J.D.; Labbé, C.; Rycroft, D.S.; Okorie, D.A.; Taylor, D.A.H. Tetranortriterpenoids and related compounds. Part 23. Complex tetranortriterpenoids from Trichilia prieuriana and Guarea thompsonii (Meliaceae), and the hydrolysis products of dregeanin, prieurianin, and related compounds. J. Chem. Res. 1979, 8, 256–257. [Google Scholar]
- Polonsky, J.; Varon, Z.; Marazano, C.; Arnoux, B.; Pettit, G.R.; Schmid, J.M.; Ochi, M.; Kotsuki, H. The structure of amoorastatone and the cytotoxic limonoid 12-hydroxyamoorastatin. Experientia 1979, 35, 987–989. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, D.A.; Naidoo, N. Limonoids from Aphanamixis polystacha. Phytochemistry 1999, 51, 927–930. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, F.; Wu, D.; Chen, Q. Complete assignments of 1H and 13C NMR data for rings A,B-seco limonoids from the seed of Aphanamixis polystachya. Magn. Reson. Chem. 2007, 45, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Lidert, Z.; Taylor, D.A.H.; Thirugnanam, M. Insect antifeedant activity of four prieurianin-type limonoids. J. Nat. Prod. 1985, 48, 843–845. [Google Scholar] [CrossRef]
- Nganso Ditchou, Y.O.; Kahouo Doutsing, A.; Simo Nemg, F.B.; Nyasse, B. Chemical Constituents of Root from Turraeanthus africanus (Meliaceae) and in Vitro Antimicrobial Activity. Int. J. Innov. Stud. Sci. Engineer. Technol. 2019, 5, 36–44. [Google Scholar]
- Tsamo, A.; Langat, M.K.; Nkounga, P.; Kamden Waffo, A.F.; Nkengfack, A.E.; Mulholland, D.A. Limonoids from the West African Trichilia welwitschia (Meliaceae). Biochem. System. Ecol. 2013, 50, 368–370. [Google Scholar] [CrossRef]
- Dzoyem, J.P.; Tsamo, A.T.; Melong, R.; Mkounga, P.; Nkengfack, A.E.; McGaw, L.J.; Eloff, J.N. Cytotoxicity, nitric oxide and acetylcholinesterase inhibitory activity of three limonoids isolated from Trichilia welwitschii (Meliaceae). Biol. Res. 2015, 48, 57. [Google Scholar] [CrossRef]
- Fan, W.; Fan, L.; Wang, Z.; Yang, L. Limonoids from the Genus Melia (Meliaceae): Phytochemistry, Synthesis, Bioactivities, Pharmacokinetics, and Toxicology. Front. Pharmacol. 2022, 12, 795565. [Google Scholar] [CrossRef]
- Mulani, F.A.; Nandikol, S.S.; Thulasiram, H.V. Chemistry and Biology of Novel Meliaceae Limonoids. ChemRXiv 2022. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiAgOa7xcaDAxVnVqQEHZFIBtsQFnoECBoQAQ&url=https%3A%2F%2Fchemrxiv.org%2Fengage%2Fapi-gateway%2Fchemrxiv%2Fassets%2Forp%2Fresource%2Fitem%2F629aedbb80f81c39c69bcff3%2Foriginal%2Fchemistry-and-biology-of-novel-meliaceae-limonoids.pdf&usg=AOvVaw1QSsMnVYlkA4_4CKybEiPQ&opi=89978449 (accessed on 18 February 2024).
- Kablan, A.; Saunders, R.A.; Szkudlarek-Mikho, M.; Chin, A.J.; Bosio, R.M.; Fujii, K.; Shapiro, J.; Chin, K.V. Prieurianin Causes Weight Loss in Diet-Induced Obese Mice and Inhibits Adipogenesis in Cultured Preadipocytes. J. Diabetes Metab. 2010, 1, 1000101. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Sun, D.; Bai, F. Protective Effect of Nimbolide against High Fat Diet-induced Obesity in Rats via Nrf2/HO-1 Pathway. J. Oleo. Sci. 2022, 71, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Ono, E.; Inoue, J.; Hashidume, T.; Shimizu, M.; Sato, R. Anti-obesity and anti-hyperglycemic effects of the dietary citrus limonoid nomilin in mice fed a high-fat diet. Biochem. Biophys. Res. Commun. 2011, 410, 677–681. [Google Scholar] [CrossRef]
- Sato, R. Nomilin as an anti-obesity and anti-hyperglycemic agent. Vitam. Horm. 2013, 91, 425–439. [Google Scholar]
- Shen, Y.; Hao, X. Natural product sciences: An integrative approach to the innovations of plant natural products. Sci. China Life Sci. 2020, 63, 1634–1650. [Google Scholar] [CrossRef] [PubMed]
- Najmi, A.; Javed, S.A.; Al Bratty, M.; Alhazmi, H.A. Modern Approaches in the Discovery and Development of Plant-Based Natural Products and Their Analogues as Potential Therapeutic Agents. Molecules 2022, 27, 349. [Google Scholar] [CrossRef]
- Robert, S.; Chary, S.N.; Drakakaki, G.; Li, S.; Yang, Z.; Raikhel, N.V.; Hicks, G.R. Endosidin1 defines a compartment involved in endocytosis of the brassinosteroid receptor BRI1 and the auxin transporters PIN2 and AUX1. Proc. Natl. Acad. Sci. USA 2008, 105, 8464–8469. [Google Scholar] [CrossRef]
- Tóth, R.; Gerding-Reimers, C.; Deeks, M.J.; Menninger, S.; Gallegos, R.M.; Tonaco, I.A.; Hübel, K.; Hussey, P.J.; Waldmann, H.; Coupland, G. Prieurianin/endosidin 1 is an actin-stabilizing small molecule identified from a chemical genetic screen for circadian clock effectors in Arabidopsis thaliana. Plant J. 2012, 71, 338–352. [Google Scholar] [CrossRef]
- Anuradha, A.; Annadurai, R.S.; Shashidhara, L.S. Actin cytoskeleton as a putative target of the neem limonoid Azadirachtin A. Insect. Biochem. Mol. Biol. 2007, 37, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Pravin Kumar, R.; Manoj, M.N.; Kush, A.; Annadurai, R.S. In silico approach of azadirachtin binding with actins. Insect. Biochem. Mol. Biol. 2007, 37, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Pravin Kumar, R.; Roopa, L.; Sudheer Mohammed, M.M.; Kulkarni, N. Azadirachtin(A) distinctively modulates subdomain 2 of actin—Novel mechanism to induce depolymerization revealed by molecular dynamics study. J. Biomol. Struct. Dyn. 2016, 34, 2698–2710. [Google Scholar]
- Woollard, A.A.; Moore, I. The functions of Rab GTPases in plant membrane traffic. Curr. Opin. Plant Biol. 2008, 11, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zheng, H. Rab-A1c GTPase defines a population of the trans-Golgi network that is sensitive to endosidin1 during cytokinesis in Arabidopsis. Mol. Plant 2013, 6, 847–859. [Google Scholar] [CrossRef]
- Qi, X.; Zheng, H. Functional analysis of small Rab GTPases in cytokinesis in Arabidopsis thaliana. Methods Mol. Biol. 2013, 1043, 103–112. [Google Scholar]
- Wang, J.; Bai, M.; Zhang, C.; An, N.; Wan, L.; Wang, X.N.; Du, R.H.; Shen, Y.; Yuan, Z.Y.; Wu, X.D.; et al. Natural compound fraxinellone ameliorates intestinal fibrosis in mice via direct intervention of HSP47-collagen interaction in the epithelium. Acta Pharmacol. Sin. 2023, 44, 2469–2478. [Google Scholar] [CrossRef]
- Yoneda, A.; Minomi, K.; Tamura, Y. HSP47 promotes metastasis of breast cancer by interacting with myosin IIA via the unfolded protein response transducer IRE1α. Oncogene 2020, 39, 4519–4537. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Smieško, M.; Sellner, M.; Lill, M.A. Decision Making in Structure-Based Drug Discovery: Visual Inspection of Docking Results. J. Med. Chem. 2021, 64, 2489–2500. [Google Scholar] [CrossRef] [PubMed]
- Agu, P.C.; Afiukwa, C.A.; Orji, O.U.; Ezeh, E.M.; Ofoke, I.H.; Ogbu, C.O.; Ugwuja, E.I.; Aja, P.M. Molecular docking as a tool for the discovery of molecular targets of nutraceuticals in diseases management. Sci. Rep. 2023, 13, 13398. [Google Scholar] [CrossRef] [PubMed]
- Bellone, M.L.; Muñoz Camero, C.; Chini, M.G.; Dal Piaz, F.; Hernandez, V.; Bifulco, G.; De Tommasi, N.; Braca, A. Limonoids from Guarea guidonia and Cedrela odorata: Heat Shock Protein 90 (Hsp90) Modulator Properties of Chisomicine D. J. Nat. Prod. 2021, 84, 724–737. [Google Scholar] [CrossRef] [PubMed]
- Braga, T.M.; Rocha, L.; Chung, T.Y.; Oliveira, R.F.; Pinho, C.; Oliveira, A.I.; Morgado, J.; Cruz, A. Biological Activities of Gedunin-A Limonoid from the Meliaceae Family. Molecules 2020, 25, 493. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Haque, E.; Hameed, R.; Maier, P.N.; Irfan, S.; Kamil, M.; Nazir, A.; Mir, S.S. Hsp90 inhibitor gedunin causes apoptosis in A549 lung cancer cells by disrupting Hsp90:Beclin-1:Bcl-2 interaction and downregulating autophagy. Life Sci. 2020, 256, 118000. [Google Scholar] [CrossRef] [PubMed]
- Bailly, C.; Vergoten, G. Interaction of Camptothecin Anticancer Drugs with Ribosomal Proteins L15 and L11: A Molecular Docking Study. Molecules 2023, 28, 1828. [Google Scholar] [CrossRef]
- Vergoten, G.; Bailly, C. Molecular Docking of Cryptoconcatones to α-Tubulin and Related Pironetin Analogues. Plants 2023, 12, 296. [Google Scholar] [CrossRef]
- Widmer, C.; Gebauer, J.M.; Brunstein, E.; Rosenbaum, S.; Zaucke, F.; Drögemüller, C.; Leeb, T.; Baumann, U. Molecular basis for the action of the collagen-specific chaperone Hsp47/SERPINH1 and its structure-specific client recognition. Proc. Natl. Acad. Sci. USA 2012, 109, 13243–13247. [Google Scholar] [CrossRef]
- Zheng, W.W.; Yang, D.T.; Wang, J.X.; Song, Q.S.; Gilbert, L.I.; Zhao, X.F. Hsc70 binds to ultraspiracle resulting in the upregulation of 20-hydroxyecdsone-responsive genes in Helicoverpa armigera. Mol. Cell. Endocrinol. 2010, 315, 282–291. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, F.X.; Cai, M.J.; Zhao, W.L.; Li, X.R.; Wang, J.X.; Zhao, X.F. The hormone-dependent function of Hsp90 in the crosstalk between 20-hydroxyecdysone and juvenile hormone signaling pathways in insects is determined by differential phosphorylation and protein interactions. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 5184–5192. [Google Scholar] [CrossRef] [PubMed]
- Nojima, Y. Characterization of Heat Shock Protein 60 as an Interacting Partner of Superoxide Dismutase 2 in the Silkworm, Bombyx mori, and Its Response to the Molting Hormone, 20-Hydroxyecdysone. Antioxidants 2021, 10, 1385. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Ye, Y.; Zheng, Z.W.; Luo, W.; Gong, Y.J.; Feng, Q.L.; Li, S.; Huang, L.H. Cytoplasmic Hsp70s promote EcR transport into the nucleus by responding to various stimuli. Insect Biochem. Mol. Biol. 2023, 157, 103964. [Google Scholar] [CrossRef] [PubMed]
- Scudeler, E.L.; Garcia, A.S.G.; Padovani, C.R.; Dos Santos, D.C. Pest and natural enemy: How the fat bodies of both the southern armyworm Spodoptera eridania and the predator Ceraeochrysa claveri react to azadirachtin exposure. Protoplasma 2019, 256, 839–856. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lai, D.; Yuan, M.; Xu, H. Growth inhibition and differences in protein profiles in azadirachtin-treated Drosophila melanogaster larvae. Electrophoresis 2014, 35, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Camero, C.M.; Vassallo, A.; De Leo, M.; Temraz, A.; De Tommasi, N.; Braca, A. Limonoids from Aphanamixis polystachya Leaves and Their Interaction with Hsp90. Planta Med. 2018, 8, 964–970. [Google Scholar] [CrossRef]
- Su, Y.; Li, X.; Zhang, W.; Zhang, B.; Ma, C.S. Dynamics of heat shock protein responses to thermal stress changes after metamorphosis in a lepidopteran insect. Arch. Insect Biochem. Physiol. 2021, 107, e21791. [Google Scholar] [CrossRef]
- King, A.M.; MacRae, T.H. Insect heat shock proteins during stress and diapause. Annu. Rev. Entomol. 2015, 60, 59–75. [Google Scholar] [CrossRef]
- Wang, S.; Kuperman, L.L.; Song, Z.; Chen, Y.; Liu, K.; Xia, Z.; Xu, Y.; Yu, Q. An overview of limonoid synthetic derivatives as promising bioactive molecules. Eur. J. Med. Chem. 2023, 259, 115704. [Google Scholar] [CrossRef] [PubMed]
- Berson, T.; von Wangenheim, D.; Takáč, T.; Šamajová, O.; Rosero, A.; Ovečka, M.; Komis, G.; Stelzer, E.H.; Šamaj, J. Trans-Golgi network localized small GTPase RabA1d is involved in cell plate formation and oscillatory root hair growth. BMC Plant Biol. 2014, 14, 252. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.; Zhou, J.; Faulkner, C.; MacLean, D.; Robatzek, S. Spatio-temporal cellular dynamics of the Arabidopsis flagellin receptor reveal activation status-dependent endosomal sorting. Plant Cell. 2012, 24, 4205–4219. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, N.; Okuno, D.; Tokito, T.; Yura, H.; Kido, T.; Ishimoto, H.; Tanaka, Y.; Mukae, H. HSP47: A Therapeutic Target in Pulmonary Fibrosis. Biomedicines 2023, 11, 2387. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Fattah, E.E.; Zakaria, A.Y. Targeting HSP47 and HSP70: Promising therapeutic approaches in liver fibrosis management. J. Transl. Med. 2022, 20, 544. [Google Scholar] [CrossRef] [PubMed]
- Bellaye, P.S.; Burgy, O.; Bonniaud, P.; Kolb, M. HSP47: A potential target for fibrotic diseases and implications for therapy. Expert Opin. Ther. Targets 2021, 25, 49–62. [Google Scholar] [CrossRef]
- Miyamura, T.; Sakamoto, N.; Kakugawa, T.; Taniguchi, H.; Akiyama, Y.; Okuno, D.; Moriyama, S.; Hara, A.; Kido, T.; Ishimoto, H.; et al. Small molecule inhibitor of HSP47 prevents pro-fibrotic mechanisms of fibroblasts in vitro. Biochem. Biophys. Res. Commun. 2020, 530, 561–565. [Google Scholar] [CrossRef]






| Compounds | Plants 1 | Plant Parts Used | Reported Activities | References |
|---|---|---|---|---|
| Aphanamixinin | A. polystachya | Bark | No activity reported. | [56,57] |
| Aphanamixoids A–B | A. polystachya | Leaves and twigs | Moderate antifeedant activity against the beet armyworm (Spodoptera exigua). | [58] |
| Aphanamixoids K–P | A. polystachya | Leaves and twigs | Weak antifeedants against the lepidopteran agricultural pest Helicoverpa armigera. | [51] |
| Aphanaonoids A–H Aphanaonoids I–J | A. polystachya A. sinensis | Leaves and twigs | No activity reported. | [52] |
| Aphapolynins A–B | A. polystachya | Fruits | Modest antiproliferative activity of aphapolynin A against two carcinoma cell lines. | [59] |
| Aphapolynins C–I | A. polystachya | Fruits | Aphapolynin C displayed moderate activity against the phytopathogenic fungus Pythium dissimile and insecticidal effects. | [46] |
| Ciparasin P | C. cinerascens | Leaves | Significant anti-HIV activity and little cytotoxicity against MT-4 cells. | [60] |
| Dysoxylumin A | D. mollissimum | Twigs | Cytotoxic activity against A549 cancer cell. | [61] |
| Dysoxylumins A–C | D. hainanense | Bark | No activity reported. | [62] |
| Monbasone, monbasol | T. mombasana | Roots | No activity reported. | [63] |
| Munronoid O | M. unifoliolata | Whole plant | Inhibition of TMV infection. | [64] |
| Muronin A | M. henryi | Twigs | Cytotoxic activity against cancer cell lines. | [65] |
| Muronin O, P, Q | M. henryi | Twigs | Antiviral effect against the tobacco mosaic virus (TMV). | [66,67] |
| Munronin T, U | M. henryi | Twigs | Weak protection from tobacco mosaic virus (TMV) infection. | [68] |
| Munropins A–F | M. pinnata | Aerial parts | No cytotoxic activity. | [69] |
| Trichavensin | T. havanensis | Seeds | No activity reported. | [70] |
| Trichilianones A–D | T. adolfi | Bark | Weak antiparasitic activity against Leishmania braziliensis promastigotes in vitro. | [71] |
| Trichilones A–E | T. adolfi | Bark | Weak cytotoxic activity. | [72] |
| Trichirokin | T. emetica | Stem bark | No antibacterial or cytotoxic activity. | [73] |
| Zaphaprinins A–Y | A. grandifolia | Fruits | Marked insecticidal activity of zaphaprinins I and R against the grain aphid Sitobion avenae and the diamondback moth Plutella xylostella. | [55] |
| Compounds | CID * | ΔE (kcal/mol) | ΔG (kcal/mol) |
|---|---|---|---|
| Fraxinellone | 124039 | −41.70 | −19.80 |
| Prieurianin | 329486 | −106.50 | −30.60 |
| Rohitukin | 99982 | −88.50 | −34.45 |
| Dregeanin | 433157 | −92.25 | −19.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergoten, G.; Bailly, C. Insights into the Mechanism of Action of the Degraded Limonoid Prieurianin. Int. J. Mol. Sci. 2024, 25, 3597. https://doi.org/10.3390/ijms25073597
Vergoten G, Bailly C. Insights into the Mechanism of Action of the Degraded Limonoid Prieurianin. International Journal of Molecular Sciences. 2024; 25(7):3597. https://doi.org/10.3390/ijms25073597
Chicago/Turabian StyleVergoten, Gérard, and Christian Bailly. 2024. "Insights into the Mechanism of Action of the Degraded Limonoid Prieurianin" International Journal of Molecular Sciences 25, no. 7: 3597. https://doi.org/10.3390/ijms25073597