Phylogenomic Study of Monechma Reveals Two Divergent Plant Lineages of Ecological Importance in the African Savanna and Succulent Biomes
Abstract
:1. Introduction
1.1. Ecological Importance of Members of Monechma s.l.
1.2. Aims of the Present Study
2. Materials and Methods
2.1. Sampling
2.2. DNA Isolation and Sequencing Methods
2.3. Phylogenetic Reconstruction
2.4. Phylogenetic Analyses
2.5. Hypothesis Testing
2.6. Divergence Time Estimation
2.7. Biome Evolution and Climatic Niche
2.8. Morphological Studies
3. Results
3.1. Phylogenetic Results
3.2. Divergence Times
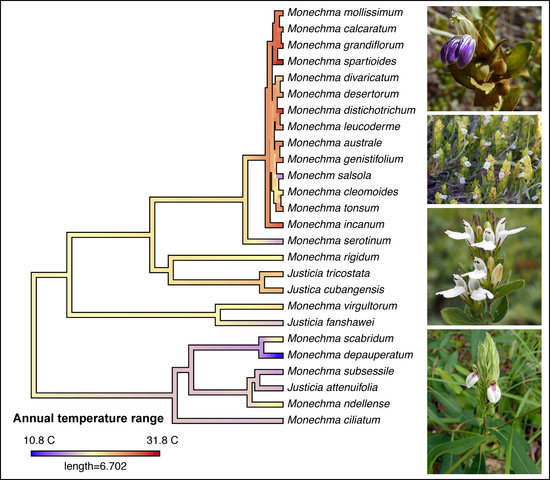
3.3. Biome Evolution and Climatic Niche
3.4. Taxonomically Informative Morphological Traits
4. Discussion
4.1. Ecology and Biogeography of Monechma Groups I and II
4.2. Taxonomic Implications
4.2.1. Morphological and Cytological Traits for the Separation of Monechma Groups I and II
4.2.2. Relationship of Monechma Group I to Justicia sect. Harnieria
4.2.3. Morphological Variation within Monechma Group II
4.2.4. The Status of Monechma varians and Justicia platysepala
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Darbyshire, I.; Kordofani, M.; Farag, I.; Candiga, R.; Pickering, H. The Plants of Sudan and South Sudan: An Annotated Checklist; Royal Botanic Gardens, Kew: Richmond, UK, 2015. [Google Scholar]
- Darbyshire, I.; Timberlake, J.; Osborne, J.; Rokni, S.; Matimele, H.; Langa, C.; Datizua, C.; de Sousa, C.; Alves, T.; Massingue, A.; et al. The endemic plants of Mozambique: Diversity and conservation status. PhytoKeys 2019, 136, 45–96. [Google Scholar] [CrossRef] [PubMed]
- Onana, J.M. The Vascular Plants of Cameroon. A taxonomic checklist with IUCN assessments. In Flore du Cameroon; National Herbarium of Cameroon: Yaoundé, Cameroon, 2011; Volume 39. [Google Scholar]
- Gosline, G.; Cheek, M.; Bidault, E.; van der Burgt, X.M.; Challen, G.; Couch, C.; Couvreur, T.; Darbyshire, I.; Dawson, S.; Goyder, D.; et al. Known plant diversity of the Republic of Guinea increased by 30% in a new taxonomically curated checklist. Unpublished work.
- Vollesen, K. Blepharis (Acanthaceae): A Taxonomic Revision; Royal Botanic Gardens, Kew: Richmond, UK, 2000. [Google Scholar]
- Tripp, E.A.; Tsai, Y.H.E.; Zhuang, Y.; Dexter, K. RADseq dataset with 90% missing data fully resolves recent radiation of Petalidium (Acanthaceae) in the ultra-arid deserts of Namibia. Ecol. Evol. 2017, 7, 7920–7936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darbyshire, I.; Tripp, E.A.; Chase, F.M. A taxonomic revision of Acanthaceae tribe Barlerieae in Angola and Namibia. Part 1. Kew Bull. 2019, 74, 1–85. [Google Scholar] [CrossRef] [Green Version]
- Darbyshire, I. Taxonomic notes and novelties in the genus Isoglossa (Acanthaceae) from east Africa. Kew Bull. 2009, 64, 401–427. [Google Scholar] [CrossRef]
- Tripp, E.A.; Dexter, K.G. Taxonomic novelties in Namibian Ruellia (Acanthaceae). Syst. Bot. 2012, 37, 1023–1030. [Google Scholar] [CrossRef]
- Darbyshire, I.; Luke, Q. Barleria mirabilis (Acanthaceae): A remarkable new tree species from west Tanzania. Kew Bull. 2016, 71, 13. [Google Scholar] [CrossRef]
- McDade, L.A.; Daniel, T.F.; Kiel, C.A. Towards a comprehensive understanding of phylogenetic relationships among lineages of Acanthaceae s.l. (Lamiales). Am. J. Bot. 2008, 95, 1136–1152. [Google Scholar] [CrossRef] [PubMed]
- McDade, L.A.; Daniel, T.F.; Kiel, C.A. The Tetramerium Lineage (Acanthaceae, Justicieae) revisited: Phylogenetic relationships reveal polyphyly of many New World genera accompanied by rampant evolution of floral morphology. Syst. Bot. 2018, 43, 97–116. [Google Scholar] [CrossRef]
- Kiel, C.A.; Daniel, T.F.; Darbyshire, I.; McDade, L.A. Unraveling relationships in the morphologically diverse and taxonomically challenging “justicioid” lineage (Acanthaceae: Justicieae). Taxon 2017, 66, 645–674. [Google Scholar] [CrossRef]
- Kiel, C.A.; Daniel, T.F.; McDade, L.A. Phylogenetics of New World ‘justicioids’ (Justicieae: Acanthaceae): Major lineages, morphological patterns, and widespread incongruence with classification. Syst. Bot. 2018, 43, 459–484. [Google Scholar] [CrossRef]
- Darbyshire, I.; Kiel, C.A.; Daniel, T.F.; McDade, L.A.; Luke, W.R.Q. Two new genera of Acanthaceae from tropical Africa. Kew Bull. 2019, 74, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Tripp, E.A.; Darbyshire, I.; Daniel, T.F.; Kiel, C.A.; McDade, L.A. Revised classification of Acanthaceae including treatment of previously unplaced genera and worldwide dichotomous keys. Unpublished work.
- Vollesen, K. Justicia . In Flora of Tropical East Africa; Beentje, H., Ed.; Royal Botanic Gardens, Kew: Richmond, UK, 2010; pp. 495–601. [Google Scholar]
- Vollesen, K. Justicia . In Flora Zambesiaca; Timberlake, J.R., Martins, E.S., Eds.; Royal Botanic Gardens, Kew: Richmond, UK, 2015; Volume 8, pp. 162–224. [Google Scholar]
- Hedrén, M. Justicia tetrasperma sp. nov.: A linking species between Justicia and Monechma (Acanthaceae). Nordic J. Bot. 1990, 10, 149–153. [Google Scholar] [CrossRef]
- Munday, J. Monechma . In Flora of Southern Africa; Leistner, O.A., Ed.; National Botanical Institute: Pretoria, South Africa, 1995; Volume 30, pp. 47–61. [Google Scholar]
- Hedberg, I.I.; Ensermu Kelbessa, E.S.; Sebsebe Demissew, P.E. (Eds.) Ensermu Kelbessa Acanthaceae. In Flora of Ethiopia & Eritrea; The National Herbarium, Addis Ababa University: Addis Ababa, Ethiopia; The Department of Systematic Botany, Uppsala University: Uppsala, Sweden, 2006; Volume 5, pp. 345–495. [Google Scholar]
- Darbyshire, I.; Goyder, D.J. Notes on Justicia sect. Monechma (Acanthaceae) in Angola, including two new species. Blumea 2019, 64, 97–107. [Google Scholar] [CrossRef]
- Dressler, S.; Schmidt, M.; Zizka, G. African Plants—A Photo Guide; Forschungsinstitut Senckenberg: Frankfurt/Main, Germany, 2014; Available online: www.africanplants.senckenberg.de (accessed on 26 February 2020).
- Munday, J. The distribution of Monechma (Acanthaceae) species in southern Africa. Bothalia 1983, 14, 575–578. [Google Scholar] [CrossRef] [Green Version]
- Ringelberg, J.J.; Zimmermann, N.E.; Weeks, A.; Lavin, M.; Hughes, C.E. Biomes as evolutionary arenas: Convergence and conservatism in the trans-continental succulent biome. Glob. Ecol. Biogeogr. 2020. [Google Scholar] [CrossRef] [Green Version]
- Schrire, B.D.; Lavin, M.A.T.T.; Lewis, G.P. Global distribution patterns of the Leguminosae: Insights from recent phylogenies. Biol. Skr. 2005, 55, 375–422. [Google Scholar]
- Klaassen, E.; Kwembeya, E. (Eds.) A Checklist of Namibian Indigenous and Naturalised Plants; Occasional Contributions No. 5; National Botanical Research Institute: Windhoek, Namibia, 2013. [Google Scholar]
- Chase, F.M. Flora of Namibia: Monechma. Unpublished work.
- White, F. Vegetation of Africa: A Descriptive Memoir to Accompany the UNESCO/AETFAT/UNSO Vegetation Map of Africa; UNESCO: Paris, France, 1983. [Google Scholar]
- Daniel, T.F.; Tripp, E.A. Louteridium (Acanthaceae: Acanthoideae: Ruellieae: Trichantherinae): Taxonomy, phylogeny, reproductive biology, and conservation. Proc. Calif. Acad. Sci. Ser. 4 2018, 65, 41–106. [Google Scholar]
- Tripp, E.A.; Darbyshire, I. Mcdadea: A new genus of Acanthaceae endemic to the Namib Desert of Southwestern Angola. Syst. Bot. 2020, 45, 200–211. [Google Scholar] [CrossRef]
- Comito, R.P. A RADseq Phylogeny of Barleria (Acanthaceae) Resolves Fine-Scale Relationships. Master’s Thesis, California State University, Long Beach, CA, USA, 2019. [Google Scholar]
- Kiel, C.A.; Tripp, E.A.; Fisher, A.E.; McDade, L.A. Is pollen involved too? Floral traits and pollinators in New World Justicia (Acanthaceae). In Proceedings of the Conference Presentation, Botany 2019 Conference, Tucson, AZ, USA, 27–31 July 2019; Available online: https://2019.botanyconference.org/engine/search/index.php?func=detail&aid=770 (accessed on 30 April 2020).
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small amounts of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Parchman, T.L.; Gompert, Z.; Mudge, J.; Schilkey, F.D.; Benkman, C.W.; Buerkle, C.A. Genome-wide association genetics of an adaptive trait in lodgepole pine. Mol. Ecol. 2012, 21, 2991–3005. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data; Babraham Institute: Cambridge, UK, 2017; Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 26 February 2020).
- Eaton, D.A.R. PyRAD: Assembly of de novo RADseq loci for phylogenetic analysis. Bioinformatics 2014, 30, 1844–1849. [Google Scholar] [CrossRef] [PubMed]
- Eaton, D.A.R.; Overcast, I. ipyrad: Interactive assembly and analysis of RADseq datasets. Bioinformatics 2020. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Posada, D.; Kozlov, A.M.; Stamatakis, A.; Benoit, M.; Flouri, T. ModelTest-NG: A new and scalable tool for the selection of DNA and protein evolutionary models. Mol. Biol. Evol. 2020, 37, 291–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eaton, D.A.R.; Spriggs, E.L.; Park, B.; Donoghue, M.J. Misconceptions on missing data in RAD-seq phylogenetics with a deep-scale example from flowering plants. Syst. Biol. 2017, 66, 399–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chifman, J.; Kubatko, L. Quartet inference from SNP data under the coalescent model. Bioinformatics 2014, 30, 3317–3324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimodaira, H. An approximately unbiased test of phylogenetic tree selection. Syst. Biol. 2002, 51, 492–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis, Version 2.72; 2009. Available online: http://mesquiteproject.org (accessed on 26 February 2020).
- Paradis, E. Molecular dating of phylogenies by likelihood methods: A comparison of models and a new information criterion. Mol. Phylogenet. Evol. 2013, 67, 436–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: http://www.R-project.org/ (accessed on 15 April 2020).
- Smith, S.A.; O’Meara, B.C. treePL: Divergence time estimation using penalized likelihood for large phylogenies. Bioinformatics 2012, 28, 2689–2690. [Google Scholar] [CrossRef] [PubMed]
- Tripp, E.A.; McDade, L.A. A rich fossil record yields calibrated phylogeny for Acanthaceae (Lamiales) and evidence for marked biases in timing and directionality of intercontinental disjunctions. Syst. Biol. 2014, 63, 660–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mautino, L.R. Nuevas especies de palinomorfos de las formaciones San José y Chiquimil (Mioceno Medio y Superior), noroeste de Argentina. Rev. Bras. Paleontol. 2011, 14, 279–290. [Google Scholar] [CrossRef]
- Graham, V.A.W. Delimitation and infra-generic classification of Justicia (Acanthaceae). Kew Bull. 1988, 43, 551–624. [Google Scholar] [CrossRef]
- Burgess, N.; Hales, J.A.; Underwood, E.; Dinerstein, E.; Olson, D.; Itoua, I.; Schipper, J.; Ricketts, T.; Newman, K. Terrestrial Ecoregions of Africa and Madagascar: A Conservation Assessment; Island Press: Washington, DC, USA, 2004. [Google Scholar]
- Beaulieu, J.M.; O’Meara, B.C.; Donoghue, M.J. Identifying hidden rate changes in the evolution of a binary morphological character: The evolution of plant habit in campanulid angiosperms. Syst. Biol. 2013, 62, 725–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagel, M. Inferring evolutionary processes from phylogenies. Zool. Scr. 1997, 26, 331–348. [Google Scholar] [CrossRef]
- Lewis, P.O. A likelihood approach to estimating phylogeny from discrete morphological character data. Syst. Biol. 2001, 50, 913–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posada, D.; Buckley, T.R. Model selection and model averaging in phylogenetics: Advantages of Akaike Information Criterion and Bayesian approaches over likelihood ratio tests. Syst. Biol. 2004, 53, 793–808. [Google Scholar] [CrossRef] [PubMed]
- WorldClim. Maps, Graphs, Tables, and Data of the Global Climate. Available online: http://worldclim.org (accessed on 15 April 2020).
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling R Package. 2014. Available online: https://rdrr.io/cran/raster/ (accessed on 15 April 2020).
- Revell, L.J. phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 2012, 3, 217–223. [Google Scholar] [CrossRef]
- Felsenstein, J. Phylogenies and the comparative method. Am. Nat. 1985, 125, 1–15. [Google Scholar] [CrossRef]
- Revell, L.J. Two new graphical methods for mapping trait evolution on phylogenies. Methods Ecol. Evol. 2013, 4, 754–759. [Google Scholar] [CrossRef]
- Thiers, B. Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden’s Virtual Herbarium. Continuously Updated. Available online: http://sweetgum.nybg.org/science/ih/ (accessed on 26 February 2020).
- JSTOR Global Plants. Available online: https://plants.jstor.org/ (accessed on 26 February 2020).
- Brummitt, R.K. World Geographical Scheme for Recording Plant Distributions, 2nd ed.; International Working Group on Taxonomic Databases for Plant Sciences (TDWG), Hunt Institute for Botanical Documentation, Carnegie Mellon University: Pittsburgh, PA, USA, 2001; Available online: http://www.tdwg.org/standards/109 (accessed on 26 February 2020).
- Immelman, K.L. Justicia . In Flora of Southern Africa; Leistner, O.A., Ed.; National Botanical Institute: Pretoria, South Africa, 1995; Volume 30, Part 3; pp. 18–46. [Google Scholar]
- Beerling, D.J.; Osborne, C.P. The origin of the savanna biome. Glob. Chang. Biol. 2006, 12, 2023–2031. [Google Scholar] [CrossRef]
- Edwards, E.J.; Osborne, C.P.; Strömberg, C.A.; Smith, S.A.; C4 Grasses Consortium. The origins of C4 grasslands: Integrating evolutionary and ecosystem science. Science 2010, 328, 587–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurin, O.; Davies, T.J.; Burrows, J.E.; Daru, B.H.; Yessoufou, K.; Muasya, A.M.; Van der Bank, M.; Bond, W.J. Savanna fire and the origins of the ‘underground forests’ of Africa. New Phytol. 2014, 204, 201–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eiserhardt, W.L.; Couvreur, T.L.; Baker, W.J. Plant phylogeny as a window on the evolution of hyperdiversity in the tropical rainforest biome. New Phytol. 2017, 214, 1408–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ackerly, D.D. Adaptation, niche conservatism, and convergence: Comparative studies of leaf evolution in the California chaparral. Am. Nat. 2004, 163, 654–671. [Google Scholar] [CrossRef] [PubMed]
- Stebbins, G.L. Aridity as a stimulus to plant evolution. Am. Nat. 1952, 86, 33–44. [Google Scholar] [CrossRef]
- Goudie, A. Namib Sand Sea: Large dunes in an ancient desert. In Geomorphological Landscapes of the World; Migon, P., Ed.; Springer: Dordrecht, Germany, 2009; pp. 163–169. [Google Scholar]
- Scotland, R.W.; Vollesen, K. Classification of Acanthaceae. Kew Bull. 2000, 55, 513–589. [Google Scholar] [CrossRef]
- Miège, J.; Josserand, N. Nombres chromosomiques d’èspeces africaines et malgaches. Candollea 1972, 27, 283–292. [Google Scholar]
- Hedrén, M. Justicia sect. Harnieria (Acanthaceae) in tropical Africa. Acta Univ. Upsal. Symb. Bot. Upsal. 1989, 29, 1–141. [Google Scholar]
- Daniel, T.F.; Chuang, T.I. Chromosome numbers of New World Acanthaceae. Syst. Bot. 1993, 18, 283–289. [Google Scholar] [CrossRef]
- Daniel, T.F. Chromosome numbers of miscellaneous Malagasy Acanthaceae. Brittonia 2006, 58, 291–300. [Google Scholar] [CrossRef]
- Grant, W.F. A cytogenetic study in the Acanthaceae. Brittonia 1955, 8, 121–149. [Google Scholar] [CrossRef]
- Darbyshire, I.; Vollesen, K. Ensermu Kelbessa Acanthaceae (Part 2). In Flora of Tropical East Africa; Beentje, H.J., Ed.; Royal Botanic Gardens, Kew: Richmond, UK, 2010. [Google Scholar]
- McDade, L.A.; Daniel, T.F.; Darbyshire, I.; Kiel, C.A. Justicieae II: Resolved placement of many genera and recognition of a new lineage sister to Isoglossinae. Unpublished work.








| Taxon | Source Specimen | Country | Latitude | Longitude |
|---|---|---|---|---|
| Dicliptera maculata Nees subsp. usambarica (Lindau) I. Darbysh. | Kiel et al. 157 (RSA) | Kenya | −0.1791 | 35.6317 |
| Dicliptera paniculata (Forssk.) I. Darbysh. | Kiel et al. 166 (RSA) | Kenya | −2.6910 | 38.1639 |
| Hypoestes forskaolii (Vahl) R. Br. | Kiel et al. 144 (RSA) | Kenya | −1.8087 | 37.5864 |
| Hypoestes triflora (Forssk.) Roem. & Schult. | Kiel et al. 151 (RSA) | Kenya | −0.7033 | 36.4346 |
| Justicia anagalloides (Nees) T. Anderson | Kiel et al. 174 (RSA) | Kenya | −3.4144 | 38.4262 |
| Justicia attenuifolia Vollesen | Golding et al. 8 (K) | Mozambique | −12.1739 | 37.5494 |
| Justicia cordata (Nees) T. Anderson | Kiel et al. 159 (RSA) | Kenya | −2.5514 | 37.8933 |
| Justicia cubangensis I. Darbysh. & Goyder | Goyder et al. 8068 (K) | Angola | −14.5897 | 16.9072 |
| Justicia eminii Lindau | Bidgood et al. 930 (K) | Tanzania | −7.9167 | 35.6000 |
| Justicia fanshawei Vollesen | Smith et al. 2010 (K) | Zambia | −9.8529 | 28.9441 |
| Justicia flava (Forssk.) Vahl | Kiel et al. 146 (RSA) | Kenya | −1.8082 | 37.5765 |
| Justicia heterocarpa T. Anderson | Kiel et al. 158 (RSA) | Kenya | −1.2745 | 36.8146 |
| Justicia kirkiana T. Anderson | Kiel et al. 177 (RSA) | Kenya | −3.8407 | 38.6681 |
| Justicia odora (Forssk.) Lam. | Tripp et al. 4073 (COLO) | Namibia | −17.6041 | 12.8872 |
| Justicia phyllostachys C.B. Clarke | Bidgood et al. 6871 (K) | Tanzania | −6.7833 | 32.0667 |
| Justicia platysepala (S. Moore) P.G. Mey. | Tripp and Dexter 4119 (COLO) | Namibia | −22.3833 | 18.4073 |
| Justicia platysepala (S. Moore) P.G. Mey. | Tripp et al. 6907 (COLO) | Angola | −12.8929 | 13.4947 |
| Justicia platysepala (S. Moore) P.G. Mey. | Tripp et al. 6919 (COLO) | Angola | −14.9700 | 12.9040 |
| Justicia pseudorungia Lindau | Kiel et al. 185 (RSA) | Kenya | −3.2222 | 40.1218 |
| Justicia sp. B. of Flora Zambesiaca | Bester 11112 (K) | Mozambique | −18.5622 | 34.8731 |
| Justicia striata (Klotzsch) Bullock | Kiel et al. 145 (RSA) | Kenya | −1.8082 | 37.5765 |
| Justicia tetrasperma Hedrén | Kahurananga et al. 2582 (K) | Tanzania | −6.1994 | 30.3536 |
| Justicia tricostata Vollesen | Bidgood et al. 5606 (K) | Tanzania | −8.4500 | 31.4833 |
| Justicia tricostata Vollesen | Gillis 11441 (RSA) | Zambia | −15.5470 | 28.2472 |
| Justicia unyorensis S. Moore | Kiel et al. 163 (RSA) | Kenya | −2.5514 | 37.8933 |
| Justicia vagabunda Benoist | Tripp et al. 1544 (RSA) | China | 21.9449 | 101.2735 |
| Kenyacanthus ndorensis (Schweinf.) I. Darbysh. & C.A. Kiel | Luke et al. 17084 (K) | Kenya | −0.1499 | 37.0238 |
| Monechma australe P.G. Mey. | Tripp et al. 2028 (RSA) | Namibia | −23.7117 | 17.2600 |
| Monechma bracteatum Hochst. | Kiel et al. 161 (RSA) | Kenya | −2.5514 | 37.8933 |
| Monechma bracteatum Hochst. | Friis et al. 13545 (K) | Ethiopia | 11.5285 | 35.1075 |
| Monechma calcaratum Hochst. | Tripp and Dexter 2043 (RSA) | Namibia | −25.8755 | 17.7929 |
| Monechma ciliatum Hochst. ex Nees | Merklinger 2013-9-55 (K) | Senegal | 15.3181 | −16.7758 |
| Monechma cleomoides C.B. Clarke | Klaassen et al. 2530 (K) | Namibia | −21.2978 | 15.2803 |
| Monechma cleomoides C.B. Clarke | Tripp et al. 1995 (RSA) | Namibia | −17.8023 | 12.3261 |
| Monechma cleomoides C.B. Clarke | Tripp et al. 1960 (RSA) | Namibia | −19.8212 | 14.1870 |
| Monechma cleomoides C.B. Clarke | Tripp et al. 1999 (RSA) | Namibia | −17.5193 | 12.2674 |
| Monechma debile Nees | Friis et al. 10459 (K) | Ethiopia | 13.8167 | 39.5500 |
| Monechma debile Nees | Kiel et al. 173 (RSA) | Kenya | −3.3496 | 38.4483 |
| Monechma depauperatum C.B. Clarke | Etuge 4446r (K) | Cameroon | 5.0833 | 9.7167 |
| Monechma desertorum C.B. Clarke | Oliver et al. 6379 (K) | Namibia | −27.4028 | 17.3833 |
| Monechma distichotrichum P.G. Mey. | Tripp et al. 2067 (RSA) | Namibia | −28.0878 | 19.5131 |
| Monechma distichotrichum P.G. Mey. | Tripp et al. 2072 (RSA) | Namibia | −27.9074 | 17.6788 |
| Monechma divaricatum C.B. Clarke | Tripp and Dexter 808 (RSA) | Namibia | −18.7071 | 17.2921 |
| Monechma divaricatum C.B. Clarke | Tripp and Dexter 783 (RSA) | Namibia | −19.5546 | 17.7329 |
| Monechma divaricatum C.B. Clarke | McDade et al. 1275 (RSA) | South Africa | −22.8833 | 29.6667 |
| Monechma divaricatum C.B. Clarke | Tripp et al. 1970 (RSA) | Namibia | −19.6156 | 13.2550 |
| Monechma divaricatum C.B. Clarke | Tripp et al. 2023 (RSA) | Namibia | −23.3475 | 17.0788 |
| Monechma divaricatum C.B. Clarke | Tripp et al. 1961 (RSA) | Namibia | −19.8429 | 14.1279 |
| Monechma divaricatum C.B. Clarke | Tripp et al. 2029 (RSA) | Namibia | −23.7117 | 17.2600 |
| Monechma divaricatum C.B. Clarke | Tripp et al. 2039 (RSA) | Namibia | −26.4395 | 18.1855 |
| Monechma divaricatum C.B. Clarke | Tripp and Dexter 4800 (COLO) | Namibia | −20.3351 | 17.5604 |
| Monechma genistifolium C.B. Clarke | Tripp and Dexter 775 (RSA) | Namibia | −21.9340 | 16.6867 |
| Monechma genistifolium C.B. Clarke | Wanntorp & Wanntorp 339 (K) | Namibia | −21.5125 | 16.0314 |
| Monechma grandiflorum Schinz | Tripp and Dexter 2034 (RSA) | Namibia | −24.3024 | 17.8223 |
| Monechma incanum C.B. Clarke | Mott 1124 (K) | Botswana | −23.7656 | 22.8097 |
| Monechma incanum C.B. Clarke | Puff 780416-2/2 (RSA) | South Africa | −27.9471 | 22.6925 |
| Monechma leucoderme C.B. Clarke | Tripp and Dexter 2044 (RSA) | Namibia | −25.8755 | 17.7929 |
| Monechma leucoderme C.B. Clarke | Tripp et al. 2083 (RSA) | Namibia | −26.2326 | 16.5967 |
| Monechma mollissimum (Nees) P.G. Mey. | Balkwill et al. 11787 (RSA) | South Africa | −28.9489 | 18.2433 |
| Monechma mollissimum (Nees) P.G. Mey. | Tripp et al. 2071 (RSA) | Namibia | −27.9231 | 17.7338 |
| Monechma monechmoides (S. Moore) Hutch. | Aiyambo et al. 323 (K) | Namibia | −19.4713 | 17.7469 |
| Monechma monechmoides (S. Moore) Hutch. | Tripp and Dexter 785 (RSA) | Namibia | −19.4713 | 17.7469 |
| Monechma monechmoides (S. Moore) Hutch. | Bingham 11019 (K) | Zambia | −15.1667 | 27.1667 |
| Monechma ndellense (Lindau) J. Miège & Heine | Harris & Fay 2150 (K) | C.A.R. | 9.1667 | 23.2167 |
| Monechma rigidum S. Moore | Goyder 8210 (K) | Angola | −12.5683 | 16.4931 |
| Monechma salsola C.B. Clarke | Klaassen et al. 2537 (K) | Namibia | −19.2528 | 14.0044 |
| Monechma salsola C.B. Clarke | Klaassen et al. 2544 (K) | Namibia | −19.1944 | 13.0861 |
| Monechma salsola C.B. Clarke | Tripp and Dexter 6934 (COLO) | Angola | −14.5999 | 12.3703 |
| Monechma scabridum S. Moore | Congdon 584 (K) | Zambia | −11.1664 | 24.1850 |
| Monechma serotinum P.G. Mey. | Tripp et al. 4066 (COLO) | Namibia | −17.5117 | 12.9696 |
| Monechma spartioides (T. Anderson) C.B. Clarke | Tripp et al. 2064 (RSA) | Namibia | −28.0878 | 19.5131 |
| Monechma sp. | Tripp and Dexter 834 (RSA) | Namibia | −17.6070 | 12.9523 |
| Monechma subsessile C.B. Clarke | Bidgood et al. 6793 (K) | Tanzania | −6.6167 | 31.9333 |
| Monechma tonsum P.G. Mey. | Nyatoro et al. 29 (K) | Namibia | −18.1367 | 13.8953 |
| Monechma tonsum P.G. Mey. | Tripp and Dexter 813 (RSA) | Namibia | −18.9546 | 16.6243 |
| Monechma varians C.B. Clarke | Synge WC437 (K) | Malawi | −10.3500 | 33.8833 |
| Monechma virgultorum S. Moore | Goyder 8471 (K) | Angola | −13.8519 | 18.2589 |
| Hypothesis | logL | D (logL) | Reject? | p-Value |
|---|---|---|---|---|
| H0. Monechma s.l. is not monophyletic | −1201,860.278 | |||
| H1. Monechma s.l. (excluding M. varians) is monophyletic | −1209,690.731 | 7830.5 | Yes | <0.0001 |
| H0. Justicia sect. Harnieria is not monophyletic | −1201,860.278 | |||
| H1. Justicia sect. Harnieria is monophyletic, i.e., Monechma Group I is not embedded within this section | −1201,862.068 | 1.7897 | No | 0.393 |
| H0. M. ciliatum is a member of Monechma Group II | −1201,860.278 | |||
| H1. M. ciliatum is not a member of Monechma Group II | −1208,510.036 | 6649.8 | Yes | <0.0001 |
| H0. M. cleomoides + M. tonsum is not monophyletic | −1201,860.278 | |||
| H1. M. cleomoides including M. tonsum is monophyletic | −1203,255.026 | 1394.7 | Yes | <0.0001 |
| H0. M. cleomoides is not monophyletic | −1201,860.278 | |||
| H1. M. cleomoides is monophyletic | −1204,005.563 | 2145.3 | Yes | <0.0001 |
| H0. M. tonsum is not monophyletic | −1201,860.278 | |||
| H1. M. tonsum is monophyletic | −1203,255.026 | 1394.7 | Yes | <0.0001 |
| Clade | Constituent Species | Distribution |
|---|---|---|
| Monechma Group I | ^Monechma bracteatum Hochst. | Africa: ANG, BOT, ERI, ETH, KEN, MLW, MOZ, NAM, NAT, SOM, SUD, TAN, TVL, UGA, ZAI, ZAM, ZIM; Asia: IND, OMA, YEM |
| ^Monechma debile (Forssk.) Nees | Africa: DJI, ERI, ETH, KEN, SOM, SUD, TAN; Asia: SAU, YEM | |
| ^Monechma monechmoides (S. Moore) Hutch. | Africa: ANG, BOT, MLW, MOZ, NAM, TVL, ZAM, ZIM | |
| Justicia carnosa Hedrén | Africa: SOM | |
| ^Justicia eminii Lindau | Africa: BUR, MLW, RWA, TAN, UGA, ZAI, ZAM | |
| ^Justicia tetrasperma Hedrén | Africa: TAN, ZAI, ZAM | |
| ^Justicia sp. B of Flora Zambesiaca | Africa: MOZ | |
| Justicia sp. C of Flora Zambesiaca | Africa: ZAM | |
| Monechma Group II | ||
| “ciliatum/scabridum” clade | ^Monechma ciliata (Jacq.) Milne-Redh. | Africa: BEN, BKN, BUR, CAF, CHA, CMN, GAM, GHA, GNB, GUI, ETH, IVO, MLI, MLW, NGA, NGR, RWA, SEN, SIE, SUD, SOSUD, TAN, TOG, UGA, ZAI, ZAM |
| ^Monechma depauperatum (T. Anderson) C.B. Clarke | Africa: BEN, CAF, CMN, GHA, GNB, GUI, IVO, MLI, NGA, SEN, SIE, SOSUD, TOG, ZAI | |
| ^Monechma ndellense (Lindau) J. Miège & Heine | Africa: BKN, CAF, GHA, GUI, MLI, SEN, SUD, TOG | |
| ^Monechma scabridum (S. Moore) C.B. Clarke | Africa: ANG, ZAI, ZAM | |
| ^Monechma subsessile (Oliv.) C.B. Clarke | Africa: ANG, BUR, KEN, RWA, TAN, UGA, ZAI, ZAM, ZIM | |
| ^Justicia attenuifolia Vollesen | Africa: MOZ, TAN | |
| “virgultorum” clade | ^Monechma virgultorum S. Moore | Africa: ANG |
| ^Justicia fanshawei Vollesen | Africa: ZAM | |
| “tricostatum” clade | Monechma glaucifolium S. Moore | Africa: ANG |
| Monechma lolioides (S. Moore) C.B. Clarke | Africa: ANG | |
| ^Monechma rigidum S. Moore | Africa: ANG | |
| ^Justicia cubangensis I. Darbysh. & Goyder | Africa: ANG | |
| Justicia eriniae I. Darbysh. | Africa: ANG | |
| Justicia laeta S. Moore | Africa: ANG | |
| ^Justicia tricostata Vollesen | Africa: TAN, ZAM | |
| “serotinum” clade | ^Monechma serotinum P.G. Mey. | Africa: NAM |
| “cleomoides” clade | ^Monechma australe P.G. Mey. | Africa: CPP, NAM |
| ^Monechma calcaratum Schinz | Africa: NAM | |
| Monechma callothamnum Munday | Africa: NAM | |
| ^Monechma cleomoides (S. Moore) C.B. Clarke | Africa: ANG, NAM | |
| Monechma crassiusculum P.G. Mey. | Africa: NAM | |
| ^Monechma desertorum (Engl.) C.B. Clarke | Africa: NAM | |
| ^Monechma divaricatum (Nees) C.B. Clarke | Africa: ANG, BOT, CPP, CPV, MOZ, NAM, NAT, OFS, SWZ, TVL, ZAM, ZIM | |
| ^Monechma distichotrichum (Lindau) P.G. Mey. | Africa: CPP, NAM | |
| ^Monechma genistifolium (Engl.) C.B. Clarke | Africa: NAM | |
| ^Monechma grandiflorum Schinz | Africa: NAM | |
| ^Monechma incanum (Nees) C.B. Clarke | Africa: BOT, CPP, NAM, OFS | |
| ^Monechma leucoderme (Schinz) C.B. Clarke | Africa: NAM | |
| ^Monechma mollissimum (Nees) P.G. Mey. | Africa: CPP, NAM | |
| Monechma robustum Bond | Africa: CPP | |
| ^Monechma salsola (S. Moore) C.B. Clarke | Africa: ANG, NAM | |
| Monechma saxatile Munday | Africa: CPP | |
| ^Monechma spartioides (T. Anderson) C.B. Clarke | Africa: CPP, NAM | |
| ^Monechma tonsum P.G. Mey. | Africa: NAM | |
| Monechma Group II incertae sedis | Justicia kasamae Vollesen | Africa: ZAM |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darbyshire, I.; Kiel, C.A.; Astroth, C.M.; Dexter, K.G.; Chase, F.M.; Tripp, E.A. Phylogenomic Study of Monechma Reveals Two Divergent Plant Lineages of Ecological Importance in the African Savanna and Succulent Biomes. Diversity 2020, 12, 237. https://doi.org/10.3390/d12060237
Darbyshire I, Kiel CA, Astroth CM, Dexter KG, Chase FM, Tripp EA. Phylogenomic Study of Monechma Reveals Two Divergent Plant Lineages of Ecological Importance in the African Savanna and Succulent Biomes. Diversity. 2020; 12(6):237. https://doi.org/10.3390/d12060237
Chicago/Turabian StyleDarbyshire, Iain, Carrie A. Kiel, Corine M. Astroth, Kyle G. Dexter, Frances M. Chase, and Erin A. Tripp. 2020. "Phylogenomic Study of Monechma Reveals Two Divergent Plant Lineages of Ecological Importance in the African Savanna and Succulent Biomes" Diversity 12, no. 6: 237. https://doi.org/10.3390/d12060237