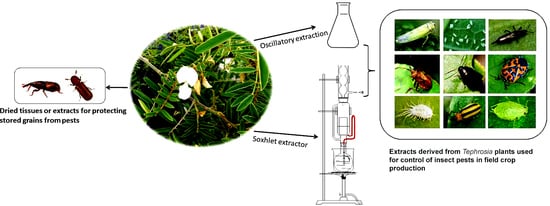
Plants in the Genus Tephrosia: Valuable Resources for Botanical Insecticides
Abstract
:Simple Summary
Abstract
1. Introduction
2. Common Species and Their Insecticidal Activities
2.1. Tephrosia vogelii
| Species | Class | Main Compound(s) | Mode of Action | Part(s) of Plant Used | Target Pests | Reference |
|---|---|---|---|---|---|---|
| Tephrosia vogelii | Rotenoid, flavonoid, and Steroid | Deguelin Tephrosin Rotenone Rotenolone α-toxicarol Elliptone Quercitin β-sitosterol Lanosterol Stigmasterol | Toxicity, larval toxicity, Antifeedant activity, Stomach or contact poison, Inhibition of oviposition, egg hatching and molting, Interference with growth and development, Interfering with the electron transport chain in mitochondria, Inhibition of cellular respiration and metabolism | Leaf Stem Root Seed Fruit coat | Acanthoscelides obtectus Aedes aegypti Aphis fabae Aphthona whitfieldi Anopheles gambiae Bactrocera curcubitea Brevicoryne brassicae Callosobruchus chinensis Callosobruchus maculatus Caryedon serratus Crocidolomia pavonana Culex quinquefasciatus Dacus cucurbitae Diabrotica undecimpunctata Euschistus heros Megalurothrips sjostedti Monolepta species Pieris rapae Phyllotreta cruciferae | [18,20,24,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48] |
| Tephrosia candida | Flavonoid and rotenoid | Candidol Dehydrorotenone 12a-hydroxyrotenone Rotenone Tephrosin Amorpholone 6a,12,-dehydodeguelin 12a-hydroxy-β-toxicarol Deguelin α-toxicarol | Stomach or contact poison, Larval toxicity, Interference with growth and development, Antifeedant activity, Inhibiting the activity of NADH oxidoreductase in electron transporters | Stem Leaf Root Seed | Aphis fabae Diaprepes abbreviates Spodoptera litura | [20,28,31,49,50,51,52,53] |
| Tephrosia elata | Flavonoid and Rotenoid | Isopongaflavone Tephrosin (S)-elatadihydrochalcone Deguelin Rotenone | Antifeedant activity, Growth inhibition activity, Inhibiting the activity of NADH, Inhibition of cellular respiration and metabolism, Larval toxicity, Inhibition of oviposition | Seed Seedpod Root | Aedes aegypti Eldana saccharina Maruca testulalis Spodoptera exempta | [20,28,54,55,56] |
| Tephrosia purpurea | Rotenoid, flavonoid, and sterol | Tephrosin Rotenone Pongaglabol Semiglabrin Quercitin Rutin (+)-tephrorins A (+)-tephrorins B β-sitosterol | Interference with growth and development, Larval toxicity, Antifeedant activity, Inhibition of cellular respiration and metabolism, Anti-inflammatory and anti-cancer properties, Inhibition of ATP production | Whole plant | Aedes aegypti Anopheles stephensi Culex quinquefasiciatus Dysdercus cingulatus Odoiporus longicollis | [20,28,57,58,59,60,61,62,63,64] |
| Tephrosia villosa | Rotenoid, sterol, and triterpenoid | Rotenone Dehydrorotenone Lupenone Stigmasterol | Larval toxicity, Stomach or contact poison, Interference with growth and development, Inhibition of oviposition Anti-parasitic | Whole plant | Anopheles gambiae Culex quinquefasciatus Tenebrio molitor Spodoptera litura | [20,47,65,66,67,68] |
| Tephrosia virginiana | Rotenoid | Rotenone Tephrosin Toxicarol | Larval toxicity, Interference with growth and development | Root | Musca domestica | [69,70] |
2.2. Tephrosia candida
2.3. Tephrosia elata
2.4. Tephrosia purpurea
2.5. Tephrosia villosa
2.6. Tephrosia virginiana
3. The Use of Tephrosia Plants for Managing Insect Pests
3.1. Commercial Formulation of Rotenone
3.2. Crude Extracts for Insect Control in Field Crop Production
3.3. Tephrosia as Cover Plant for Biocontrol of Insects and Soil Nitrogen Enrichment
3.4. Tephrosia for Controlling Insect Pests in Stored Grains
4. Concerns over the Use of Tephrosia Species as Botanical Insecticides
5. Conclusions and Future Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guedes, R.N.C.; Smagghe, G.; Stark, J.D.; Desneux, N. Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu. Rev. Entomol. 2016, 61, 43–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Carson, R. Silent Spring. Anniversary Edition; Houghton Miffin Co.: New York, NY, USA, 1962. [Google Scholar]
- Bernardes, M.F.F.; Pazin, M.; Pereira, L.C.; Dorta, D.J. Impact of pesticides on environmental and human health. In Toxicology Studies: Cells, Drugs and Environment; Andreazza, A.C., Scola, G., Eds.; InTech: Rijeka, Croatia, 2015; pp. 195–233. [Google Scholar]
- Cycon, M.; Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a strategy for the remediation of pesticide-polluted soil: A review. Chemosphere 2017, 172, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.V.R.; Proença, P.L.F.; Oliveira, J.L.; Bakshi, M.; Abhilash, P.; Fraceto, L.F. Use of botanical insecticides for sustainable agriculture: Future perspectives. Ecol. Indic. 2019, 105, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Isman, M.B. Perspective botanical insecticides: For richer, for poorer. Pest Manag. Sci. 2008, 64, 8–11. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides in the twenty—first century—fulfilling the promise? Annu. Rev. Entomol. 2020, 5, 233–249. [Google Scholar] [CrossRef] [Green Version]
- Sola, P.; Mvumi, B.M.; Ogendo, J.O.; Mponda, O.; Kamanula, J.F.; Nyirenda, S.P.; Belmain, S.R.; Stevenson, P.C. Botanical pesticide production, trade and regulatory mechanisms in sub-Saharan Africa: Making a case for plant-based pesticidal products. Food Sec. 2014, 6, 369–384. [Google Scholar] [CrossRef]
- Dougoud, J.; Toepfer, S.; Bateman, M.; Jenner, W.H. Efficacy of homemade botanical insecticides based on traditional knowledge. A review. Agron. Sustain. Dev. 2019, 39. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, N.J.; Bass, C.; Dixon, A.; Neve, P. The evolutionary origins of pesticide resistance. Biol. Rev. Camb. Philos. Soc. 2018, 94, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Siegwart, M.; Graillot, B.; Lopez, C.B.; Besse, S.; Bardin, M.; Nicot, P.C.; Lopez-Ferber, M. Resistance to bioinsecticides or how to enhance their sustainability: A review. Front. Plant Sci. 2015, 6, 381. [Google Scholar] [CrossRef] [Green Version]
- Lengai, G.M.W.; Muthomi, J.W.; Mbega, E.R. Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Sci. Afr. 2020, 7, e00239. [Google Scholar] [CrossRef]
- Hu, L.Z.; Li, X.H.; Ma, Y.Q.; Yu, X. Determination of rotenoids content in T. vogelii Hook f. Seed. J. Chin. Inst. Food Sci. Technol. 2011, 11, 206–211. [Google Scholar]
- Kariuki, D.K.; Njiru, S.N. Spectrophotometric evaluation of rotenone extraction from leaves and seeds of mature Tephrosia vogelii plant. Afr. J. Pure Appl. Chem. 2018, 12, 50–53. [Google Scholar] [CrossRef]
- Roark, R.C. Tephrosia as an Insecticide—A Review of the Literature; Roark, R.C., Ed.; U.S.Department of Agriculture, Bureau of Entomology and Plant Quarantine, Division of Insecticide Investigations, E-402: Washington, DC, USA, 1937; pp. 1–165.
- Gaskins, M.H.; White, G.A.; Martin, F.W.; Delfel, N.E.; Ruppel, E.G.; Barnes, D.K. Tephrosia vogelii: A Source of Rotenoids for Insecticidal and Piscicidal Use; Technical Bulletins from US Department of Agriculture, Economic Research Service: Washington, DC, USA, 1972. [Google Scholar] [CrossRef]
- Stevenson, P.C.; Kite, G.C.; Lewis, G.P.; Forest, F.; Nyirenda, S.P.; Belmain, S.R.; Sileshi, G.W.; Veitch, N.C. Distinct chemotypes of Tephrosia vogelii and implications for their use in pest control and soil enrichment. Phytochemistry 2012, 78, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.N.; Yan, T.; Gao, C.H.; Cao, W.H.; Huang, R.M. Natural products from the genus Tephrosia. Molecules 2014, 19, 1432–1458. [Google Scholar] [CrossRef]
- Touqeer, S.; Saeed, M.A.; Ajaib, M. A review on the phytochemistry and pharmacology of genus Tephrosia. Phytopharmacology 2013, 4, 598–637. [Google Scholar]
- Tarus, P.K.; Machocho, A.K.; Lang’at–Thoruwa, C.C.; Chhabra, S.C. Flavonoids from Tephrosia aequilata. Phytochemistry 2002, 60, 375–379. [Google Scholar] [CrossRef]
- Beentje, H. Kenya Trees, Shrubs and Lianas; National Museums of Kenya: Nairobi, Kenya, 1994; pp. 269–320. [Google Scholar]
- Morgan, E.; Wilson, I.D. Insect hormones and insect chemical ecology. In Comprehensive Natural Products Chemistry, Vol. 8: Miscellaneous Natural Products Including Marine Natural Products, Pheromones, Plant Hormones, and Aspects of Ecology; Mori, K., Ed.; Pergamon, Elsevier: Oxford, UK, 1999; pp. 263–376. [Google Scholar]
- Dzenda, T.; Ayo, J.A.; Adelaiye, A.B.; Adaudi, A.O. Ethno-medical and veterinary uses of Tephrosia vogelii Hook. F.: A review. Nig. Vet. J. 2007, 28, 24–30. [Google Scholar] [CrossRef]
- Woo, J.K.; Choi, D.S.; Tran, H.T.; Gilbert, B.E.; Hong, W.K.; Lee, H.Y. Liposomal encapsulation of deguelin: Evidence for enhanced antitumor activity in tobacco carcinogen–induced and oncogenic K-ras–induced lung tumorigenesis. Cancer Prev. Res. 2009, 2, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Murillo, G.; Salti, G.I.; Kosmeder, J.W.; Pezzuto, J.M.; Mehta, R.G. Deguelin inhibits the growth of colon cancer cells through the induction of apoptosis and cell cycle arrest. Eur. J. Cancer 2002, 38, 2446–2454. [Google Scholar] [CrossRef]
- Delfel, N.E.; Tallent, W.H.; Carlson, D.G.; Wolff, I.A. Distribution of rotenone and deguelin in Tephrosia vogelii and separation of rotenoid-rich fractions. J. Agric. Food Chem. 1970, 18, 385–390. [Google Scholar] [CrossRef]
- Ling, N. Rotenone—A review of its toxicity and use for fisheries management. Sci. Conserv. 2002, 211, 1–40. [Google Scholar]
- Kerebba, N.; Byamukama, R.; Oyedeji, A.O.; Oyedeji, O.O.; Kuria, S.K. Pesticidal activity of Tithonia diversifolia (Hemsl.) A. Gray and Tephrosia vogelii (Hook f.); phytochemical isolation and characterization: A review. S. Afr. J. Bot. 2019, 121, 366–376. [Google Scholar] [CrossRef]
- Koona, P.; Dorn, S. Extracts from Tephrosia vogelii for the protection of stored legume seeds against damage by three bruchid species. Ann. Appl. Biol. 2005, 147, 43–48. [Google Scholar] [CrossRef]
- Kayange, C.D.M.; Njera, D.; Nyirenda, S.P.; Mwamlima, L. Effectiveness of Tephrosia vogelii and Tephrosia candida extracts against common bean aphid (Aphis fabae ) in Malawi. Adv. Agric. 2019, 2019, 6704834. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Khanna, P. Production of rotenoids from Tephrosia spp. in vivo and in vitro tissue cultures. Indian J. Exp. Biol. 1975, 13, 84–85. [Google Scholar]
- Lambert, N.; Trouslot, M.F.; Nef-Campa, C.; Chrestin, H. Production of rotenoids by heterotrophic and photomixotrophic cell cultures of Tephrosia vogelii. Phytochemistry 1993, 34, 1515–1520. [Google Scholar] [CrossRef]
- Marston, A.; Msonthi, J.D.; Hostetmann, K. On the reported molluscicidal activity from Tephrosia vogelii leaves. Phytochemistry 1984, 23, 1824–1825. [Google Scholar] [CrossRef]
- Mikami, A.Y.; Ventura, M.U.; Andrei, C.C. Brown stink bug mortality by seed extracts of Tephrosia Vogelii containing deguelin and tephrosin. Braz. Arch. Biol. Technol. 2018, 61, e18180028. [Google Scholar] [CrossRef]
- Fang, N.; Casida, J.E. Anticancer action of cubé insecticide: Correlation for rotenoid constituents between inhibition of NADH: Ubiquinone oxidoreductase and induced ornithine decarboxylase activities. Proc. Natl. Acad. Sci. USA 1998, 95, 3380–3384. [Google Scholar] [CrossRef] [Green Version]
- Preston, S.; Korhonen, P.K.; Mouchiroud, L.; Cornaglia, M.; McGee, S.L.; Young, N.D.; Davis, R.A.; Crawford, S.; Nowell, C.; Ansell, B.R.E.; et al. Deguelin exerts potent nematocidal activity via the mitochondrial respiratory chain. FASEB J. 2017, 31, 4515–4532. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, J.P.; Basu, P.K. Sterols and rotenoids from fruit coat of Tephrosia vogelii Hook. Acta Hortic. 1988, 188A, 155–157. [Google Scholar]
- Belmain, S.R.; Amoah, B.A.; Nyirenda, S.P.; Kamanula, J.F.; Stevenson, P.C. Highly variable insect control efficacy of Tephrosia vogelii Chemotypes. J. Agric. Food Chem. 2012, 60, 10055–10063. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.; Freyre, R.H. Recovery of natural insecticides from Tephrosia vogelii. III. An improved procedure for sampling and assaying rotenoid content in leaves. Econ. Bot. 1967, 21, 93–98. [Google Scholar] [CrossRef]
- Fukami, J.I. Insecticides as inhibitors of respiration. In Approaches to New Leads for Insecticides; Part of The Proceedings in Life Sciences Book Series; von Keyserlingk, H.C., Jäger, A., von Szczepanski, C., Eds.; Springer: Berlin, Germany, 1976; pp. 47–69. [Google Scholar]
- Hu, L.Z.; Li, X.H.; Ma, Y.Q.; Yu, X. Study on bacteriostatic activity of extracts in different solvents from Tephrosia vogelii Hook f. seeds. Sci. Technol. Food Ind. 2011, 32, 85–88. [Google Scholar]
- Roark, R.C. A Review of the Insecticidal Uses of Rotenone and Rotenoids from Derris, Lonchocarpus (Cube and Timbo), Tephrosia, and Related Plants; United States Department of Agriculture, Bureau of Entomology and Plant Quarantine: Washington, DC, USA, 1944; pp. 2–31. [Google Scholar]
- Zeng, X.N.; Zhang, S.X.; Fang, J.F.; Han, J.Y. Comparison of the bioactivity of elliptone and rotenone against several agricultural insect pests. Kun Chong Xue Bao Acta Entomol. Sin. 2001, 45, 611–616. [Google Scholar]
- Kamal, R. Sterols from tissue cultures of Tephrosia species. Bangladesh Pharm. J. 1978, 7, 12–16. [Google Scholar]
- Samuel, V.J.; Mahesh, A.R.; Murugan, V. Phytochemical and pharmacological aspects of Tephrosia genus:A brief review. J. Appl. Pharm. Sci. 2019, 9, 117–125. [Google Scholar]
- Kidukuli, A.W.; Maregesi, S.M.; Saria, J.; Otieno, N.J.; Lawi, Y.; Nondo, R.S.; Innocent, E.M.; Mlimbila, J.; Mihale, M.J.; Moshi, M.J. Larvicidal efficacy of some Tephrosia species extracts against Anopheles Gambiae Ss and Culex Quinquefasciatus Say. Spatula DD 2015, 5, 21–25. [Google Scholar] [CrossRef]
- Alao, F.O.; Adebayo, T.A. Comparative efficacy of Tephrosia vogelii and Moringa oleifera against insect pests of watermelon (Citrullus lanatus Thumb). Int. Lett. Nat. Sci. 2015, 35, 71–78. [Google Scholar]
- Andrei, C.C.; Vieira, P.C.; Fernandes, J.B.; Da Silva MFGF. Dimethylchromene rotenoids from Tephrosia candida. Phytochemistry 1997, 46, 1081–1085. [Google Scholar] [CrossRef]
- Kole, R.K.; Satpathi, C.; Chowdhury, A.; Ghosh, M.R.; Adityachaudhury, N. Isolation of amorpholone, a potent rotenoid insecticide from Tephrosia candida. J. Agric. Food Chem. 1992, 40, 1208–1210. [Google Scholar] [CrossRef]
- Dutt, S.K.; Chibber, S.S. Candidol, a flavonol from Tephrosia candida. Phytochemistry 1983, 22, 325–326. [Google Scholar] [CrossRef]
- Roy, M.; Mitra, S.R.; Bhattacharyya, A.; Adityachaudhury, N. Candidone, a flavanone from Tephrosia candida. Phytochemistry 1986, 25, 961–962. [Google Scholar] [CrossRef]
- Parmar, V.S.; Jain, R.; Gupta, S.R.; Boll, P.M.; Mikkelsen, J.M. Phytochemical Investigation of Tephrosia candida: Hplc separation of tephrosin and 12a-hydroxyrotenone. J. Nat. Prod. 1988, 51, 185. [Google Scholar] [CrossRef]
- Bentley, M.D.; Hassanali, A.; Lwande, W.; Njoroge, P.E.W.; Sitayo, E.N.; Yatagai, M. Insect antifeedants from Tephrosia elata Deflers. Int. J. Trop. Insect Sci. 1987, 8, 85–88. [Google Scholar] [CrossRef] [Green Version]
- Muiva, L.M.; Yenesew, A.; Derese, S.; Heydenreich, M.; Peter, M.G.; Akala, H.M.; Eyase, F.; Waters, N.C.; Mutai, C.; Keriko, J.M.; et al. Antiplasmodial β-hydroxydihydrochalcone from seedpods of Tephrosia elata. Phytochem. Lett. 2009, 2, 99–102. [Google Scholar] [CrossRef]
- Mutisya, L.M. Antiplasmodial and Larvicidal Flavonoids from the Seedpods of Tephrosia Elata and Tephrosia Aequilata; Jomo Kenyatta University of Agriculture and Technology: Juja, Kenya, 2009. [Google Scholar]
- Sahayaraj, K.; Kombiah, P.; Dikshit, A.K.; Rathi, J.M. Chemical constituents of the essential oils of Tephrosia purpurea and Ipomoea carnea and their repellent activity against Odoiporus longicollis. J. Serbian Chem. Soc. 2015, 80, 465–473. [Google Scholar] [CrossRef]
- Kumar, D.; Dhamodaran, P.; Nilani, P.; Balakrishnan, N. Larvicidal activity of Tephrosia purpurea, (L) against the Larvae of Culex quinquefasiciatus. J. Appl. Pharm. Sci. 2012, 2, 219–221. [Google Scholar] [CrossRef] [Green Version]
- Rao, A.S.; Yadav, S.S.; Singh, P.; Nandal, A.; Singh, N.; Ganaie, S.A.; Yadav, N.; Kumar, R.; Bhandoria, M.S.; Bansal, P. A comprehensive review on ethnomedicine, phytochemistry, pharmacology, and toxicity of Tephrosia purpurea (L.) Pers. Phytother. Res. 2020, 34, 1902–1925. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Ali, Z.; Hussaini, S.R.; Iqbal, F.; Zahid, M.; Abbas, M.; Saba, N. Flavonoids of Tephrosia purpurea. Fitoterapia 1999, 70, 443–445. [Google Scholar] [CrossRef]
- Jain, A.; Lodhi, S.; Singhai, A.K. Simultaneous estimation of quercetin and rutin in Tephrosia purpurea Pers by high performance thin-layer chromatography. Asian J. Tradit. Med. 2009, 4, 104–109. [Google Scholar]
- Chang, L.C.; Gerhäuser, C.; Song, L.; Farnsworth, N.R.; Pezzuto, J.M.; Kinghorn, A.D. Activityguided isolation of constituents of Tephrosia purpurea with the potential to induce the phase II enzyme, quinone reductase. J. Nat. Prod. 1997, 60, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Parmar, V.S.; Rathore, J.S.; Jain, R.; Henderson, D.A.; Malone, J.F. Occurrence of pongamol as the enol structure in Tephrosia purpurea. Phytochemistry 1989, 28, 591–593. [Google Scholar] [CrossRef]
- Sahayaraj, K.; Shoba, J. Toxic effect of Tephrosia purpurea (Linn.) and Acalypha indica (Linn.) aqueous extracts impact on the mortality, macromolecules, intestinal electrolytes and detoxication enzymes of Dysdercus cingulatus (Fab.). Asian J. Biochem. 2012, 7, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Nondo, R.S.; Mbwambo, Z.H.; Kidukuli, A.W.; Innocent, E.M.; Mihale, M.J.; Erasto, P.; Moshi, M.J. Larvicidal, antimicrobial and brine shrimp activities of extracts from Cissampelos mucronata and Tephrosia villosa from coast region, Tanzania. BMC Complement. Altern. Med. 2011, 11, 33–37. [Google Scholar] [CrossRef] [Green Version]
- Vijayan, S.; Singh, N.K.; Shukla, P.; Kirti, P.B. Defensin (TvD1) from Tephrosia villosa exhibited strong anti-insect and antifungal activities in transgenic tobacco plants. J. Pest Sci. 2013, 86, 337–344. [Google Scholar] [CrossRef]
- Prashant, A.; Krupadanam, G.L.D. Dehydro-6-hydroxyrotenoid and lupenone from Tephrosia villosa. Phytochemistry 1993, 32, 484–486. [Google Scholar] [CrossRef]
- Ganapaty, S.; Nyamathulla, S.; Srilakshmi, G.V.K.; Prasad, R. Chemical and antimicrobial studies of the roots of Tephrosia villosa (L) Pers. Asian J. Chem. 2008, 20, 4498–4502. [Google Scholar]
- Little, V.A. Devil’s shoestring as an insecticide. Science 1931, 73, 315–316. [Google Scholar] [CrossRef]
- Sievers, A.F.; Lowman, M.S.; Russell, G.A.; Sullivan, W.N. Changes in the insecticidal value of the roots of cultivated devil’s shoestring, Tephrosia virginiana, at four seasonal growth periods. Amer. J. Bot. 1940, 27, 284–289. [Google Scholar] [CrossRef]
- Crombie, L.; Whiting, D.A. Biosynthesis of the rotenoid group of natural products: Applications of isotope methodology. Phytochemistry 1998, 49, 1479–1507. [Google Scholar] [CrossRef]
- Fang, N.; Casida, J.E. Cube resin insecticide: Identification and biological activity of 29 rotenoid constituents. J. Agric. Food Chem. 1999, 47, 2130–2136. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C. Rotenone. In Encyclopedia of Toxicology, 3rd ed.; Philip, W., Ed.; Academic Press; Elsevier: London, UK, 2014; pp. 185–187. [Google Scholar]
- Hegazy, M.E.F.; Mohamed, A.E.H.; El-Halawany, A.M.; Djemgou, P.C.; Shahat, A.A.; Pare, P.W. Estrogenic activity of chemical constituents from Tephrosia candida. J. Nat. Prod. 2011, 74, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, F. Toxicology of Insecticides; Plenum Press; Springer: New York, NY, USA, 1975; pp. 105–251. [Google Scholar]
- Lapointe, S.L.; McKenzie, C.L.; Hunter, W.B. Toxicity and repellency of Tephrosia candida to larva and adult Diaprepes root weevil (Coleoptera: Curculionidae). J. Econ. Entomol. 2003, 96, 811–816. [Google Scholar] [CrossRef]
- Fagerström, M.H.H.; Noordwijk, M.V.; Phien, T.; Vinh, N.C. Innovations within upland rice-based systems in northern Vietnam with Tephrosia candida as fallow species, hedgerow, or mulch: Net returns and farmers’ response. Agric. Ecosyst. Environ. 2001, 86, 23–37. [Google Scholar] [CrossRef]
- Basu, P.K.; Gupta, I. Role of Tephrosia candida DC in enhancing the amino acid content of north Bengal soil. Geobios 1988, 15, 18–21. [Google Scholar]
- Andrei, C.C.; Vieira, P.C.; Fernandes, J.B.; Silva, M.; Fo, E.R. New spirorotenoids from Tephrosia candida. Z. Naturforsch. C 2002, 57, 418–422. [Google Scholar] [CrossRef] [Green Version]
- Dalwadi, P.P.; Patel, J.L.; Patani, P.V. Tephrosia purpurea Linn (Sharpunkha, wild indigo): A review on phytochemistry and pharmacological studies. Indian J. Pharm. Biol. Res. 2014, 2, 108. [Google Scholar] [CrossRef]
- Pelter, A.; Ward, R.S.; Rao, E.V.; Raju, N.R. 8-Substituted flavonoids and 3’-substituted 7-oxygenated chalcones from Tephrosia purpurea. J. Chem. Soc. Perkin Trans. 1981, 1, 2491–2498. [Google Scholar] [CrossRef]
- Venkadachalam, R.; Subramaniyan, V.; Palani, M.; Subramaniyan, M.; Srinivasan, P.; Raji, M. Mosquito larvicidal and pupicidal activity of Tephrosia purpurea Linn. (Family: Fabaceae) and Bacillus sphaericus against, dengue vector, Aedes aegypti. Pharmacogn. J. 2017, 9, 737–742. [Google Scholar] [CrossRef] [Green Version]
- Khatoon, S.; Irshad, S.; Pandey, M.M.; Rastogi, S.; Rawat, A.K.S. A validated HPTLC densitometric method for determination of lupeol, β-sitosterol and rotenone in Tephrosia purpurea: A seasonal study. J. Chromatogr. Sci. 2019, 57, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, S.; Imani, J.; Tanneeru, K.; Guruprasad, L.; Kogel, K.H.; Kirti, P.B. Enhanced antifungal and insect a-amylase inhibitory activities of Alpha-TvD1, a peptide variant of Tephrosia villosa defensin (TvD1) generated through in vitro mutagenesis. Peptides 2012, 33, 220–229. [Google Scholar] [CrossRef]
- Gard, M. The toxicity of extracts of Tephrosia virginiana (Fabaceae) in Oklahoma. Okla. Native Plant Rec. 2010, 10, 54–64. [Google Scholar] [CrossRef] [Green Version]
- Othman, Z.S.; Hassan, N.H.; Zubairi, S.I. Imidazolium-Based Ionic Liquid Binary Solvent System as an Extraction Medium in Enhancing the Rotenone Yield Extracted from Derris elliptica Roots. In Progress and Developments in Ionic Liquids; IntechOpen: London, UK, 2017; pp. 495–515. [Google Scholar]
- Casacchia, T.; Sofo, A.; Toscano, P.; Sebastianelli, L.; Perri, E. Persistence and effects of rotenone on oil quality in two Italian olive cultivars. Food Chem. Toxicol. 2009, 47, 214–219. [Google Scholar] [CrossRef]
- Cheng, H.M.; Yamamoto, I.; Casida, J.E. Rotenone photodecomposition. J. Agric. Food Chem. 1972, 20, 850–856. [Google Scholar] [CrossRef]
- Mangum, F.A.; Madrigal, J.L. Rotenone effect on aquatic macroinvertebrates of the Strawberry River, Utah: A five-year summary. J. Freshw. Ecol. 1999, 14, 125–135. [Google Scholar] [CrossRef]
- Sherer, T.B.; Betarbet, R.; Testa, C.M.; Seo, B.B.; Richardson, J.R.; Kim, J.H.; Miller, G.W.; Yagi, T.; Matsuno-Yagi, A.; Greenamyre, J.T. Mechanism of toxicity in rotenone models of Parkinson’s disease. J. Neurosci. 2003, 23, 10756–10764. [Google Scholar] [CrossRef]
- Tanner, C.M.; Kamel, F.; Ross, G.W.; Hoppin, J.A.; Goldman, S.M.; Korell, M.; Marras, C.; Bhudhikanok, G.S.; Kasten, M.; Chade, A.R.; et al. Rotenone, Paraquat, and Parkinson’s Disease. Environ. Health Perspect. 2011, 119, 866–872. [Google Scholar] [CrossRef] [Green Version]
- Baker, B. Rotenone Use in Organic Farming. Hygeia Anal. 2017. Available online: https://hygeia-analytics.com/2017/01/04/rotenone-use-in-organic-farming/ (accessed on 20 September 2020).
- Codex Alimentarius Commission (CAC). Guidelines for the Production, Processing, Labelling and Marketing of Organically Produced Foods: Annex 2: Deletion of Rotenone (CL2008/27-fl): Government Comments at Step 3. 2009. Available online: http://www.fao.org/input/download/standards/360/cxg_032e.pdf (accessed on 10 October 2020).
- United States Department of Agriculture (USDA). Foreign Agricultural Service. China Release Newmaximum Residue Limits for Pesticides in Food. Global Agricultural Information Network. 2018. Available online: https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=China%20Releases%20Standard%20for%20Maximum%20Residue%20Limits%20in%20Foods%20_Beijing_China%20-%20Peoples%20Republic%20of_5-22-2019.pdf (accessed on 18 September 2020).
- Stevenson, P.C.; Belmain, S.R. Pesticidal plants in African agriculture: Local uses and global perspectives. Outlooks Pest Manag. 2016, 27, 226–230. [Google Scholar] [CrossRef] [Green Version]
- Tembo, Y.; Mkindi, A.G.; Mkenda, P.A.; Mpumi, N.; Mwanauta, R.; Stevenson, P.C.; Ndakidemi, P.A.; Belmain, S.R. Pesticidal plant extracts improve yield and reduce insect pests on legume crops without harming beneficial arthropods. Front. Plant Sci. 2018, 9, 1425. [Google Scholar] [CrossRef] [PubMed]
- Donno, D.; Beccaro, G.L.; Cerutti, A.K.; Mellano, M.G.; Bounous, G. Bud extracts as new phytochemical source for herbal preparations: Quality control and standardization by analytical fingerprint. In Phytochemicals—Isolation, Characterisation and Role in Human Health; Rao, V., Rao, L., Eds.; Tech: Rijeka, Croazia, 2015; pp. 187–218. [Google Scholar]
- Mkenda, P.; Mwanauta, R.; Stevenson, P.C.; Ndakidemi, P.; Mtei, K.; Belmain, S.R. Extracts from field margin weeds provide economically viable and environmentally benign pest control compared to synthetic pesticides. PLoS ONE 2015, 10, e0143530. [Google Scholar] [CrossRef] [PubMed]
- Reuben, S.O.; Masunga, M.; Makundi, R.; Misangu, R.N.; Kilonzo, B.; Mwatawala, M.; Lyimo, H.F.; Ishengoma, C.G.; Msuya, D.G.; Mulungu, L.S. Control of Cowpea Weevil (Callosobruchus maculatus L.) in Stored Cowpea (Vigna unguiculatus L.) Grains using Botanicals. Asian J. Plant Sci. 2006, 5, 91–97. [Google Scholar]
- Anjarwalla, P.; Ofori, D.A.; Jamnadass, R.; Mowo, J.G.; Stevenson, P.C. Proceedings of the Training Workshop on Sustainable Production, Harvesting and Conservation of Botanical Pesticides; World Agroforestry Centre (ICRAF): Nairobi, Kenya, 2013; Available online: http://projects.nri.org/options/images/handbook.pdf (accessed on 18 September 2020).
- Huang, J.G.; Zhou, L.J.; Xu, H.H.; An, Y.X. Recent advances in the insecticidal plant, Tephrosia vogelii. Plant Prot. 2006, 32, 18–21. [Google Scholar]
- Baligar, V.C.; Fageria, N.K. Agronomy and physiology of tropical cover crops. J. Plant Nutr. 2007, 30, 1287–1339. [Google Scholar] [CrossRef]
- Bosman, M.T.M.; DeHaas, A.J.P. A revision of the genus Tephrosia (Leguminosae-Papilionoideae) in Malesia. Blumea 1983, 28, 421–487. [Google Scholar]
- Munthali, M.G.; Gachene, C.K.K.; Sileshi, G.W.; Karanja, N.K. Amendment of Tephrosia improved fallows with inorganic fertilizers improves soil chemical properties, N uptake, and maize yield in Malawi. Int. J. Agron. 2014. [Google Scholar] [CrossRef] [Green Version]
- Hagedorn, F.; Steiner, K.G.; Sekayange, L.; Zech, W. Effect of rainfall pattern on nitrogen mineralization and leaching in a green manure experiment in South Rwanda. Plant Soil 1997, 195, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, P.C.; Arnold, S.E.J.; Belmain, S.R. Pesticidal plants for stored product pests on small-holder farms in Africa. In Advances in Plant Biopesticides; Springer: Berlin/Heidelberg, Germany, 2014; pp. 149–172. [Google Scholar] [CrossRef]
- Boeke, S.J.; van Loon, J.J.A.; van Huis, A.; Kossou, D.K.; Dicke, M. The Use of Plant Material to Protect Stored Seeds against Seed Beetles: A Review; Laboratory of Entomology, Wageningen University: Wageningen, The Netherlands, 2001; Available online: https://edepot.wur.nl/282994 (accessed on 20 September 2020).
- Ogendo, J.O.; Belmain, S.R.; Deng, A.L.; Walker, D.J. Comparison of toxic and repellent effects of Lantana Camara L. with Tephrosia vogelii hook and a synthetic pesticide against Sitophilus zeamais motschulsky (Coleoptera: Curculionidae) in stored maize grain. Int. J. Trop. Insect Sci. 2003, 23, 127–135. [Google Scholar] [CrossRef]
- Wilbaux, R. Considerations sur Tephrasia vogelii Hook. f. et uncertain hombre d’especes voisines. Ann. Gembloux. 1935, 41, 1–30. [Google Scholar]
- Irvine, J.E.; Freyre, R.H. The occurrence of rotenoids in some species of the genus Tephrosia. J. Agric. Food Chem. 1959, 7, 106–107. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [Green Version]
- Mkindi, A.G.; Tembo, Y.; Mbega, E.R.; Medvecky, B.; Kendal-Smith, A.; Farrell, I.W.; Ndakidemi, P.A.; Belmain, S.R.; Stevenson, P.C. Phytochemical analysis of Tephrosia vogelii across East Africa reveals three chemotypes that influence its use as a pesticidal plant. Plants 2019, 8, 597. [Google Scholar] [CrossRef] [Green Version]
- Ye, H.Y.; Chen, L.J.; Li, Y.F.; Peng, A.H.; Fu, A.F.; Song, H.; Tang, M.H.; Luo, H.D.; Luo, Y.F.; Xu, Y.B.; et al. Preparative isolation and purification of three rotenoids and one isoflavone from the seeds of Millettia pachycarpa Benth by high-speed counter-current chromatography. J. Chromatogr. A 2008, 1178, 101–107. [Google Scholar] [CrossRef]
- Wen, L.; Chen, Y. Molecular mechanism of deguelin in anti-tumor effect. Curr. Pharm. Anal. 2012, 8, 14–19. [Google Scholar] [CrossRef]
- Varughese, R.S.; Lam, W.S.-T.; Marican, A.A.B.H.; Viganeshwari, S.H.; Bhave, A.S.; Syn, N.L.; Wang, J.G.; Wong, A.L.-A.; Kumar, A.P.; Lobie, P.E.; et al. Biopharmacological considerations for accelerating drug development of deguelin, a rotenoid with potent chemotherapeutic and chemopreventive potential. Cancer 2019, 125, 1789–1798. [Google Scholar] [CrossRef]
- Prijono, D.; Panggraito, A.; Syahbirin, G. Variation in insecticidal activity of leaf extracts of Tephrosia vogelii J.D. Hooker (Leguminosae) from West Java, Indonesia. In Proceedings of the 3rd International Conference on Applied Life Sciences (ICALS2014), Bangi, Malaysia, 18–20 September 2014; ISALS Publishing: London, UK, 2014; pp. 1–5. [Google Scholar]
- Chen, J.; Henny, R.J. Role of micropropagation in the development of ornamental foliage plant industry. In Floriculture, Ornamental and Plant Biotechnology, V; Da Silva, J.A.T., Ed.; Global Science Books: London, UK, 2008; pp. 206–218. [Google Scholar]
- Chen, J.; Henny, R.J. Somaclonal variation: An important source for cultivar development of floriculture crops. In Floriculture, Ornamental and Plant Biotechnology, II; Da Silva, J.A.T., Ed.; Global Science Books: London, UK, 2006; pp. 244–253. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Qin, D.; Chen, J.; Zhang, Z. Plants in the Genus Tephrosia: Valuable Resources for Botanical Insecticides. Insects 2020, 11, 721. https://doi.org/10.3390/insects11100721
Zhang P, Qin D, Chen J, Zhang Z. Plants in the Genus Tephrosia: Valuable Resources for Botanical Insecticides. Insects. 2020; 11(10):721. https://doi.org/10.3390/insects11100721
Chicago/Turabian StyleZhang, Peiwen, Deqiang Qin, Jianjun Chen, and Zhixiang Zhang. 2020. "Plants in the Genus Tephrosia: Valuable Resources for Botanical Insecticides" Insects 11, no. 10: 721. https://doi.org/10.3390/insects11100721