Nutritional Relationship between Bemisia tabaci and Its Primary Endosymbiont, Portiera aleyrodidarum, during Host Plant Acclimation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect and Plants
2.2. Host Plant Shifting and Acclimation
2.3. Performance of B. tabaci
2.4. Expression of Nutrient-Related Genes of P. aleyrodidarum
2.5. Statistical Analyses
3. Results
3.1. Effects of Host Plant Shifting and Acclimation on the Performance of B. tabaci
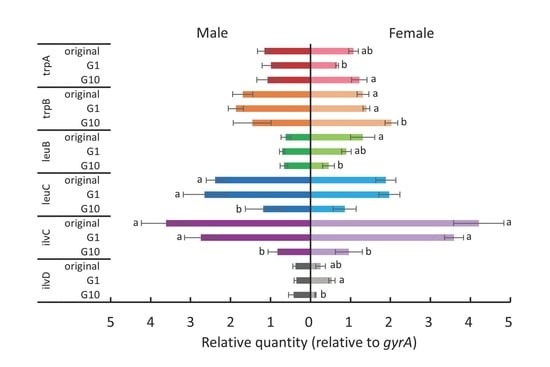
3.2. Relationship between Host Plant Shifting and Acclimation and the Expression of Nutrient-Related Genes of P. aleyrodidarum
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baumann, P. Biology of bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu. Rev. Microbiol. 2005, 59, 155–189. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. The microbial dimension in insect nutritional ecology. Funct. Ecol. 2009, 23, 38–47. [Google Scholar] [CrossRef]
- Bourtzis, K.; Miller, T.A. Insect Symbiosis, Volume 2; CRC Press: Boca Raton, FL, USA, 2006; p. 304. [Google Scholar]
- Oliver, K.M.; Russell, J.A.; Moran, N.A.; Hunter, M.S. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl. Acad. Sci. USA 2003, 100, 1803–1807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.; Vilchez, I.; Mateos, M. Spiroplasma bacteria enhance survival of Drosophila hydei attacked by the parasitic wasp Leptopilina heterotoma. PLoS ONE 2010, 5, e12149. [Google Scholar] [CrossRef] [PubMed]
- Scarborough, C.L.; Ferrari, J.; Godfray, H.C.J. Aphid protected from pathogen by endosymbiont. Science 2005, 310, 1781. [Google Scholar] [CrossRef]
- Łukasik, P.; van Asch, M.; Guo, H.; Ferrari, J.; Godfray, H.C.J. Unrelated facultative endosymbionts protect aphids against a fungal pathogen. Ecol. Lett. 2013, 16, 214–218. [Google Scholar] [CrossRef]
- Tsuchida, T.; Koga, R.; Horikawa, M.; Tsunoda, T.; Maoka, T.; Matsumoto, S.; Simon, J.C.; Fukatsu, T. Symbiotic bacterium modifies aphid body color. Science 2010, 330, 1102–1104. [Google Scholar] [CrossRef] [PubMed]
- Brumin, M.; Kontsedalov, S.; Ghanim, M. Rickettsia influences thermotolerance in the whitefly Bemisia tabaci B biotype. Insect Sci. 2011, 18, 57–66. [Google Scholar] [CrossRef]
- Wernegreen, J.J. Mutualism meltdown in insects: Bacteria constrain thermal adaptation. Curr. Opin. Microbiol. 2012, 15, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Kontsedalov, S.; Zchori-Fein, E.; Chiel, E.; Gottlieb, Y.; Inbar, M.; Ghanim, M. The presence of Rickettsia is associated with increased susceptibility of Bemisia tabaci (Homoptera: Aleyrodidae) to insecticides. Pest Manag. Sci. 2008, 64, 789–792. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Hayatsu, M.; Hosokawa, T.; Nagayama, A.; Tago, K.; Fukatsu, T. Symbiont-mediated insecticide resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 8618–8622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottlieb, Y.; Fein, E.Z.; Mozes-Daube, N.; Kontsedalov, S.; Skaljac, M.; Brumin, M.; Sobol, I.; Czosnek, H.; Vavre, F.; Fleury, F.; et al. The transmission efficiency of Tomato yellow leaf curl virus by the whitefly Bemisia tabaci is correlated with the presence of a specific symbiotic bacterium species. J. Virol. 2010, 84, 9310–9317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rana, V.S.; Singh, S.T.; Priya, N.G.; Kumar, J.; Rajagopal, R. Arsenophonus GroEL interacts with CLCuV and is localized in midgut and salivary gland of whitefly B. tabaci. PLoS ONE 2012, 7, e42168. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Pan, H.; Liu, B.; Chu, D.; Xie, W.; Wu, Q.; Wang, S.; Xu, B.; Zhang, Y. Insect symbiont facilitates vector acquisition, retention, and transmission of plant virus. Sci. Rep. 2013, 3, 1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, J.A.; Kelly, S.E.; Perlman, S.J.; Hunter, M.S. Cytoplasmic incompatibility in the parasitic wasp Encarsia inaron: Disentangling the roles of Cardinium and Wolbachia symbionts. Heredity 2009, 102, 483–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourtzis, K.; Miller, T.A. Insect Symbiosis; CRC Press: Boca Raton, FL, USA, 2003; p. 368. [Google Scholar]
- De Barro, P.J.; Liu, S.S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A statement of species status. Annu. Rev. Entomol. 2011, 56, 1–19. [Google Scholar] [CrossRef]
- Boykin, L.M.; De Barro, P.J. A practical guide to identifying members of the Bemisia tabaci species complex: And other morphologically identical species. Front. Ecol. Evol. 2014, 2, 45. [Google Scholar] [CrossRef] [Green Version]
- Alemandri, V.; Vaghi Medina, C.G.; Dumón, A.D.; Argüello Caro, E.B.; Mattio, M.F.; García Medina, S.; López Lambertini, P.M.; Truol, G. Three members of the Bemisia tabaci (Hemiptera: Aleyrodidae) cryptic species complex occur sympatrically in Argentine horticultural crops. J. Econ. Entomol. 2015, 108, 405–413. [Google Scholar] [CrossRef]
- Wang, H.L.; Lei, T.; Xia, W.Q.; Cameron, S.L.; Liu, Y.Q.; Zhang, Z.; Gowda, M.M.N.; De Barro, P.; Navas-Castillo, J.; Omongo, C.A.; et al. Insight into the microbial world of Bemisia tabaci cryptic species complex and its relationships with its host. Sci. Rep. 2019, 9, 6568. [Google Scholar] [CrossRef] [Green Version]
- Malka, O.; Santos-Garcia, D.; Feldmesser, E.; Sharon, E.; Krause-Sakate, R.; Delatte, H.; van Brunschot, S.; Patel, M.; Visendi, P.; Mugerwa, H.; et al. Species-complex diversification and host-plant associations in Bemisia tabaci: A plant-defence, detoxification perspective revealed by RNA-Seq analyses. Mol. Ecol. 2018, 27, 4241–4256. [Google Scholar] [CrossRef] [Green Version]
- Santos-Garcia, D.; Farnier, P.A.; Beitia, F.; Zchori-Fein, E.; Vavre, F.; Mouton, L.; Moya, A.; Latorre, A.; Silva, F.J. Complete genome sequence of “Candidatus Portiera aleyrodidarum” BT-QVLC, an obligate symbiont that supplies amino acids and carotenoids to Bemisia tabaci. J. Bacteriol. 2012, 194, 6654–6655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sloan, D.B.; Moran, N.A. Endosymbiotic bacteria as a source of carotenoids in whiteflies. Biol. Lett. 2012, 8, 986–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luan, J.B.; Chen, W.; Hasegawa, D.K.; Simmons, A.M.; Wintermantel, W.M.; Ling, K.S.; Fei, Z.; Liu, S.S.; Douglas, A.E. Metabolic coevolution in the bacterial symbiosis of whiteflies and related plant sap-feeding insects. Genome Biol. Evol. 2015, 7, 2635–2647. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Hasegawa, D.K.; Kaur, N.; Kliot, A.; Pinheiro, P.V.; Luan, J.; Stensmyr, M.C.; Zheng, Y.; Liu, W.; Sun, H.; et al. The draft genome of whitefly Bemisia tabaci MEAM1, a global crop pest, provides novel insights into virus transmission, host adaptation, and insecticide resistance. BMC Biol. 2016, 14, 110. [Google Scholar] [CrossRef]
- Ankrah, N.Y.D.; Luan, J.; Douglas, A.E. Cooperative metabolism in a three-partner insect-bacterial symbiosis revealed by metabolic modeling. J. Bacteriol. 2017, 199, e00872-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, A.K.; Moran, N.A. Aphid genome expression reveals host–symbiont cooperation in the production of amino acids. Proc. Natl. Acad. Sci. USA 2011, 108, 2849–2854. [Google Scholar] [CrossRef] [Green Version]
- Santos-Garcia, D.; Mestre-Rincon, N.; Zchori-Fein, E.; Morin, S. Inside out: Microbiota dynamics during host-plant adaptation of whiteflies. ISME J. 2020, 14, 847–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, S.H.; Tsai, W.S.; Kenyon, L.; Tsai, C.W. Different transmission efficiencies may drive displacement of tomato begomoviruses in the fields in Taiwan. Ann. Appl. Biol. 2015, 166, 321–330. [Google Scholar] [CrossRef]
- Ansari, P.G.; Singh, R.K.; Kaushik, S.; Krishna, A.; Wada, T.; Noda, H. Detection of symbionts and virus in the whitefly Bemisia tabaci (Hemiptera: Aleyrodidae), vector of the Mungbean yellow mosaic India virus in Central India. Appl. Entomol. Zool. 2017, 52, 567–579. [Google Scholar] [CrossRef]
- Chapman, R.F. The Insects: Structure and Function, 4th ed.; Cambridge University Press: Cambridge, UK, 1998; p. 770. [Google Scholar]
- Rocha, D.J.P.; Santos, C.S.; Pacheco, L.G.C. Bacterial reference genes for gene expression studies by RT-qPCR: Survey and analysis. Antonie Van Leeuwenhoek 2015, 108, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Yang, P.; Xu, Y.; Luo, L.; Zhu, J.; Cui, N.; Kang, L.; Cui, F. Performances of survival, feeding behavior, and gene expression in aphids reveal their different fitness to host alteration. Sci. Rep. 2016, 6, 19344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Cao, W.J.; Zhong, L.R.; Godfray, H.C.J.; Liu, X.D. Host plant determines the population size of an obligate symbiont (Buchnera aphidicola) in aphids. Appl. Environ. Microbiol. 2016, 82, 2336–2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, A.K.; Moran, N.A. The impact of microbial symbionts on host plant utilization by herbivorous insects. Mol. Ecol. 2014, 23, 1473–1496. [Google Scholar] [CrossRef] [PubMed]
- Nishikori, K.; Morioka, K.; Kubo, T.; Morioka, M. Age- and morph-dependent activation of the lysosomal system and Buchnera degradation in aphid endosymbiosis. J. Insect. Physiol. 2009, 55, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Vigneron, A.; Masson, F.; Vallier, A.; Balmand, S.; Rey, M.; Vincent-Monégat, C.; Aksoy, E.; Aubailly-Giraud, E.; Zaidman-Rémy, A.; Heddi, A. Insects recycle endosymbionts when the benefit is over. Curr. Biol. 2014, 24, 2267–2273. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Zou, C.; Ban, F.; Wang, H.; Wang, X.; Liu, Y. Conservation of transcriptional elements in the obligate symbiont of the whitefly Bemisia tabaci. PeerJ 2019, 7, e7477. [Google Scholar] [CrossRef]
- Rakha, M.; Hanson, P.; Ramasamy, S. Identification of resistance to Bemisia tabaci Genn. in closely related wild relatives of cultivated tomato based on trichome type analysis and choice and no-choice assays. Genet. Resour. Crop Evol. 2017, 64, 247–260. [Google Scholar] [CrossRef] [Green Version]
- Chuche, J.; Auricau-Bouvery, N.; Danet, J.L.; Thiéry, D. Use the insiders: Could insect facultative symbionts control vector-borne plant diseases? J. Pest Sci. 2017, 90, 51–68. [Google Scholar] [CrossRef]
- Douglas, A.E. Symbiotic microorganisms: Untapped resources for insect pest control. Trends Biotechnol. 2007, 25, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.K.; Pesko, K.N.; Quintero-Hernández, V.; Possani, L.D.; Miller, T.A.; Durvasula, R.V. A paratransgenic strategy to block transmission of Xylella fastidiosa from the glassy-winged sharpshooter Homalodisca vitripennis. BMC Biotechnol. 2018, 18, 50. [Google Scholar] [CrossRef] [Green Version]








| Gene | ID 1 | Sequence (5′ to 3′) | Amplicon |
|---|---|---|---|
| trpA | Por0185 | trpA-F: ACCACCAGAACACGCTCAAGAA trpA-R: CTTCCTCCCGTAACTCCATTTATTG | 165 bp |
| trpB | Por0186 | trpB-F: CGGGTCTAACGCAATGGGAT trpB-R: ACTCCAGGCACACCTCCATTTAA | 135 bp |
| leuB | Por0099 | leuB-F: TGGTATAGGGCCCGAAGTGATC leuB-R: AGCGAGATGCTTTTGCTGCTT | 170 bp |
| leuC | Por0263 | leuC-F: CAGATGGAGGCACAGGATATGC leuC-R: CCAACTTTTGCTCCTGCTTCAA | 115 bp |
| ilvC | Por0140 | ilvC-F: TGTGGTGGCGTATCAGCATTAA ilvC-R: TCACCCAAACCCCCTTCATATA | 140 bp |
| ilvD | Por0090 | ilvD-F: AGGCAATGGGACGTTGTTATCA ilvD-R: CCACCCATTGCTATATCCATTATCAT | 180 bp |
| gyrA | Por0266 | gyrA-F: TATGGCAACGAATGTACCTCCTC gyrA-R: ACAATCCCAGCAGTCCCATAAAT | 159 bp |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, F.-Y.; Tsai, C.-W. Nutritional Relationship between Bemisia tabaci and Its Primary Endosymbiont, Portiera aleyrodidarum, during Host Plant Acclimation. Insects 2020, 11, 498. https://doi.org/10.3390/insects11080498
Hu F-Y, Tsai C-W. Nutritional Relationship between Bemisia tabaci and Its Primary Endosymbiont, Portiera aleyrodidarum, during Host Plant Acclimation. Insects. 2020; 11(8):498. https://doi.org/10.3390/insects11080498
Chicago/Turabian StyleHu, Fang-Yu, and Chi-Wei Tsai. 2020. "Nutritional Relationship between Bemisia tabaci and Its Primary Endosymbiont, Portiera aleyrodidarum, during Host Plant Acclimation" Insects 11, no. 8: 498. https://doi.org/10.3390/insects11080498